Overview
The article centers on pivotal techniques and implementation strategies for Next-Generation Sequencing (NGS) in lung cancer, underscoring its critical role in personalizing treatment through genetic profiling. It elucidates how NGS enhances diagnostic precision, facilitates the identification of actionable mutations, and tackles challenges such as reimbursement and staff training. This comprehensive discussion highlights the transformative impact of NGS on lung cancer management, reinforcing its significance in contemporary medical practice.
Introduction
In the realm of oncology, the emergence of Next-Generation Sequencing (NGS) has heralded a new era in the diagnosis and treatment of lung cancer. This groundbreaking technology enables the simultaneous analysis of multiple genes and uncovers critical genetic alterations that can dictate treatment strategies. Given that lung cancer remains a leading cause of cancer-related mortality, leveraging NGS for personalized medicine represents a transformative shift in patient care.
From enhancing diagnostic accuracy to facilitating targeted therapies, the implications of NGS extend far beyond the laboratory, promising to reshape the landscape of lung cancer management. As the integration of these advanced sequencing techniques continues to evolve, understanding their methodologies, applications, and the challenges faced in clinical implementation becomes essential for maximizing their potential in improving patient outcomes.
Explore Next-Generation Sequencing in Lung Cancer: Key Concepts and Importance
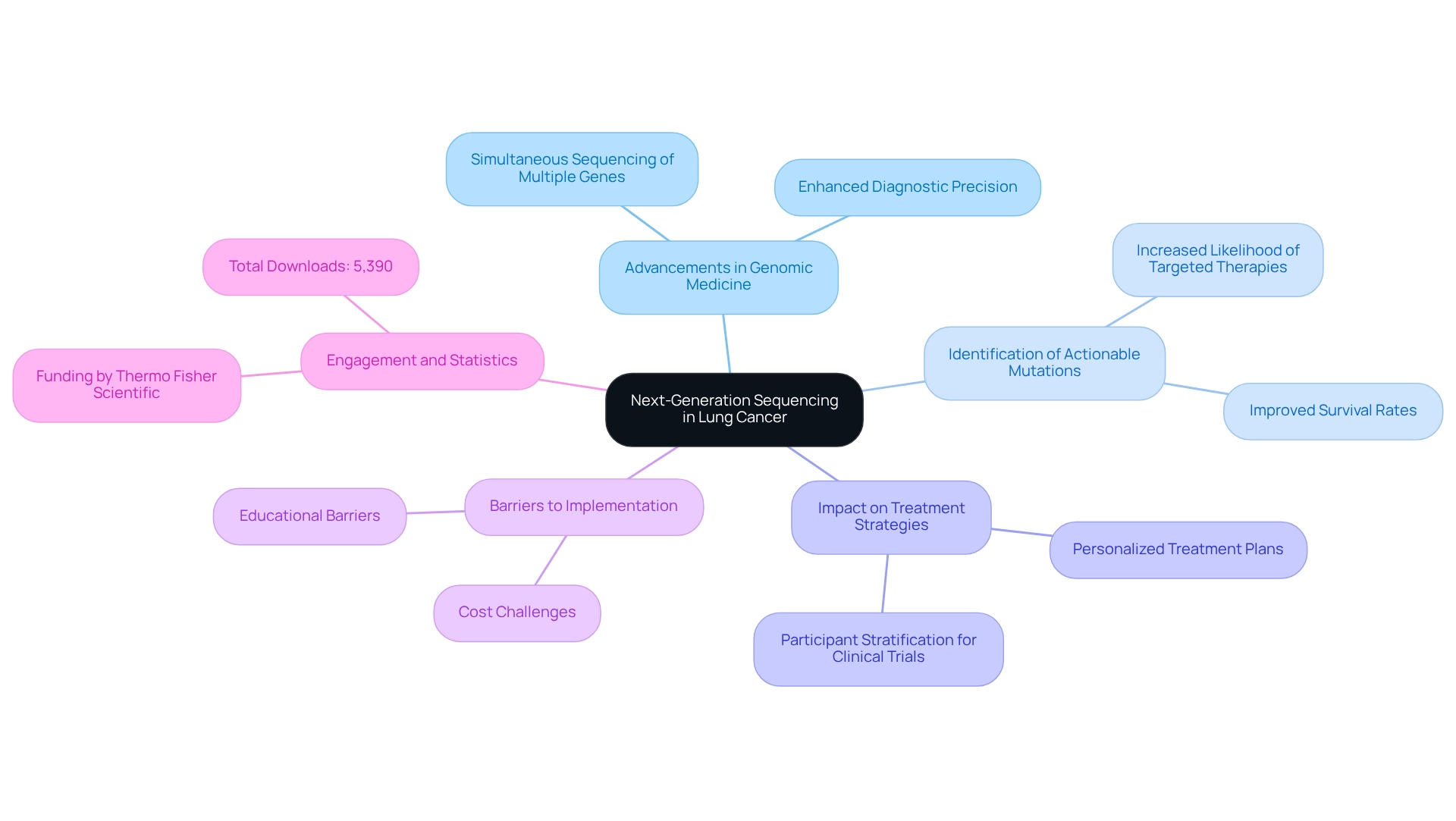
Next-Generation Sequencing (NGS) lung cancer represents a significant advancement in genomic medicine, especially in the context of respiratory tumors. This technology facilitates the simultaneous sequencing of multiple genes, offering a comprehensive overview of the genetic alterations present in tumors. It is essential for identifying actionable mutations that can direct targeted therapies, ultimately resulting in personalized treatment plans. The importance of NGS in pulmonary malignancies lies in its ability to enhance diagnostic precision, enable early mutation identification, and improve participant stratification for clinical trials. Given that NGS lung cancer remains one of the leading causes of cancer-related deaths, the application of NGS can profoundly affect healthcare management and outcomes.
Recent statistics reveal that the adoption of NGS has resulted in a marked increase in the identification of actionable mutations, which are crucial for effective treatment planning. Research indicates that individuals whose tumors are analyzed via NGS are more likely to receive targeted therapies, contributing to improved survival rates. Additionally, expert opinions underscore the necessity of addressing existing barriers, such as cost and educational challenges, to fully leverage the potential of NGS in clinical practice. As Dr. Nir Peled, MD PhD, remarked, “However, there are cost and educational barriers to the full implementation of next-generation sequencing that need to be overcome.”
Case studies further illustrate the long-term strategic growth achievable through insights derived from NGS. By providing timely and relevant information, CareSet empowers healthcare providers to optimize the lifecycle management of pharmaceutical products, ultimately enhancing patient care. The article has garnered significant attention, with a total of 5,390 downloads, reflecting the growing engagement with NGS in the treatment of respiratory tumors. As the landscape of pulmonary tumor treatment evolves, the role of NGS lung cancer will continue to expand, solidifying its position as a cornerstone of precision medicine in oncology.
Examine NGS Techniques: Methodologies for Lung Cancer Diagnosis
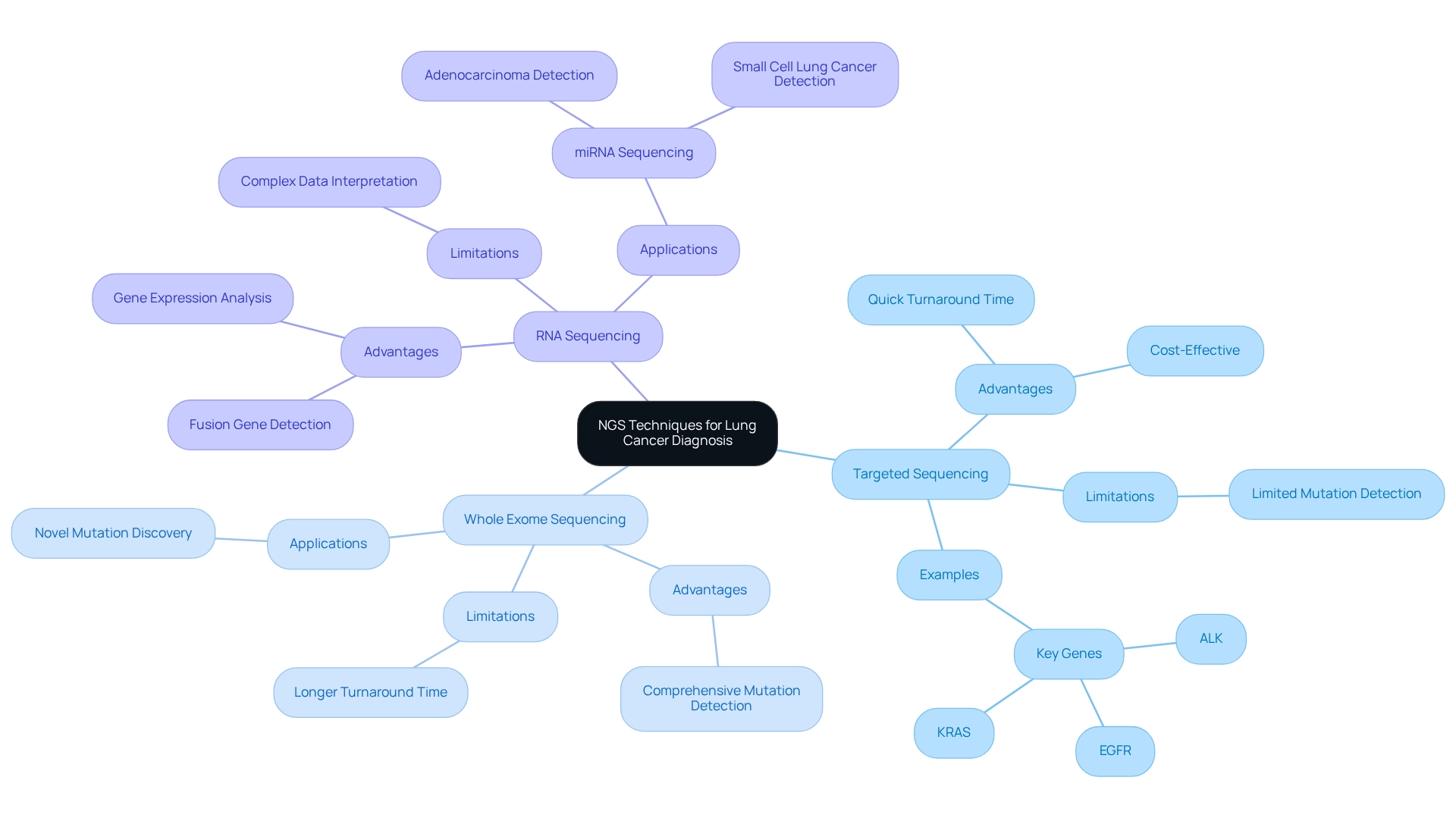
Next-generation sequencing (NGS) lung cancer techniques are pivotal in diagnosing respiratory tumors, utilizing three primary methodologies: targeted sequencing, whole exome sequencing, and RNA sequencing. Targeted sequencing concentrates on specific genes associated with respiratory tumors, including EGFR, ALK, and KRAS. This targeted approach facilitates the precise identification of mutations that can significantly impact treatment decisions, especially in the realm of targeted therapies.
Conversely, whole exome sequencing examines all coding regions of the genome, offering a comprehensive perspective that can uncover novel mutations not detected by targeted methods. This extensive analysis is particularly beneficial in scenarios where the mutation landscape remains unclear or when conventional treatments are ineffective.
RNA sequencing provides an alternative viewpoint by assessing gene expression levels and identifying fusion genes that may not be detectable through DNA sequencing alone. This methodology is essential for comprehending the functional implications of mutations and the overall biology of tumors.
Each methodology offers distinct advantages and limitations, making the selection of technique dependent on the clinical context and specific diagnostic inquiries. For example, while targeted sequencing is typically quicker and more cost-effective—boasting a turnaround time (TAT) for sequential analysis of single biomarkers estimated at approximately two weeks—whole exome sequencing may reveal additional mutations pertinent to treatment. Furthermore, the emergence of mutations such as EGFR, ALK, and KRAS in pulmonary tumors is expected to evolve, with ongoing research indicating significant implications for patient management by 2025.
Recent case studies, including investigations into miRNA sequencing for pulmonary carcinoma diagnosis, underscore the potential of these advanced techniques as non-invasive diagnostic tools. These studies have explored the application of miRNA sequencing to pinpoint specific miRNAs associated with various types of pulmonary tumors, including adenocarcinoma and small cell carcinoma (SCLC). By identifying these specific miRNAs, such approaches could enable early detection and improve outcomes for patients. As the realm of respiratory tumor diagnostics continues to advance, the integration of NGS lung cancer techniques will be vital for optimizing treatment strategies and enhancing care for patients.
Apply NGS Insights: Transforming Lung Cancer Treatment Strategies
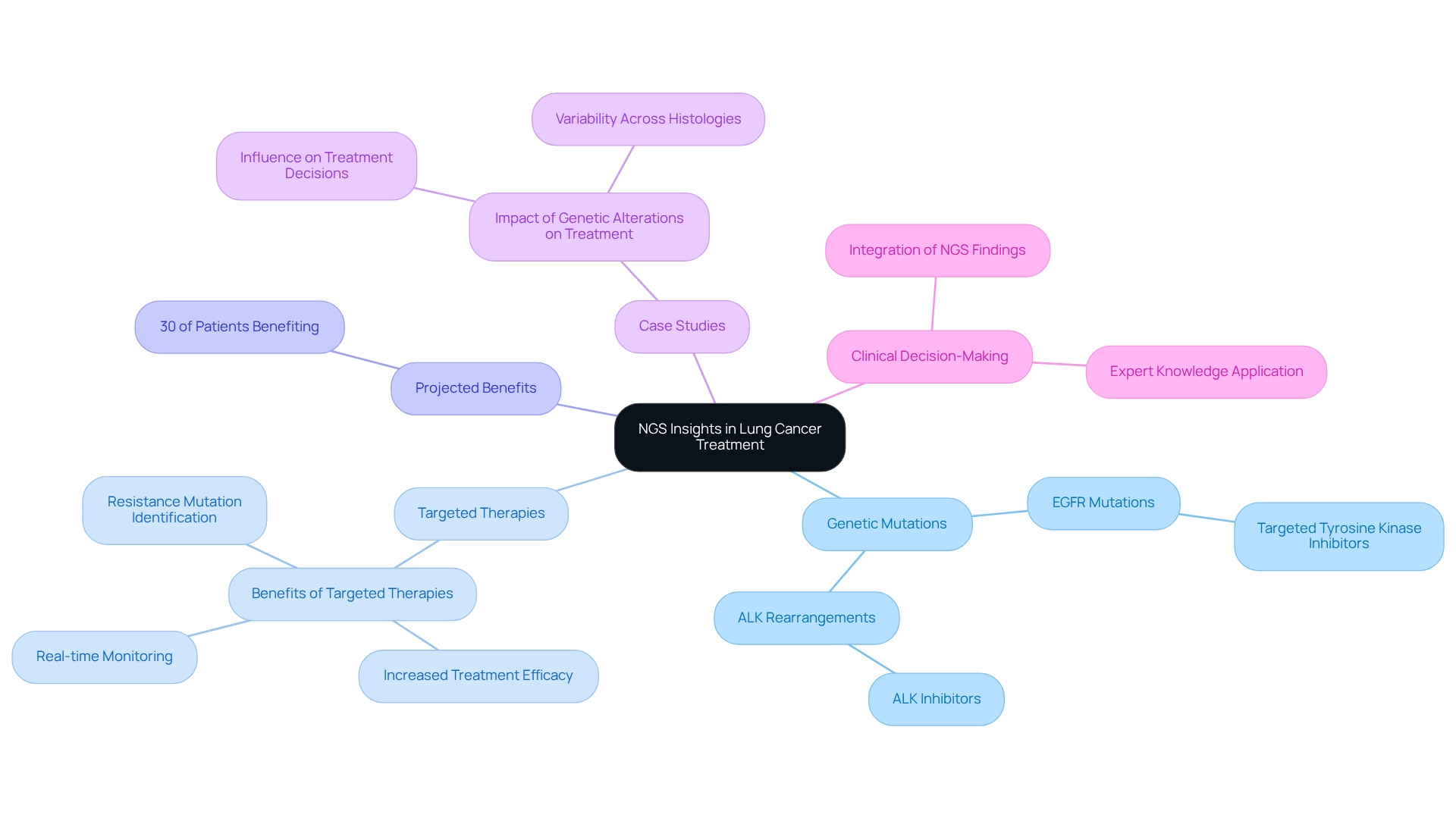
Insights from next-generation sequencing (NGS) lung cancer are revolutionizing treatment strategies for pulmonary tumors by enabling oncologists to tailor therapies based on specific genetic mutations. For instance, individuals with EGFR mutations can benefit from targeted tyrosine kinase inhibitors, while those with ALK rearrangements often respond favorably to ALK inhibitors. This precision medicine approach not only increases the likelihood of treatment efficacy but also facilitates real-time monitoring of treatment responses and the identification of resistance mutations, allowing for timely therapy adjustments.
In 2025, it is projected that approximately 30% of individuals with NGS lung cancer will derive benefits from targeted therapies informed by NGS insights, highlighting the critical role of genetic profiling in clinical practice. This projection aligns with results from a follow-up PET/CT scan conducted in January 2018, which revealed nearly total resolution of liver and adrenal metastases in individuals receiving targeted therapies, underscoring the effectiveness of these methods.
Case studies illustrate that comprehending the relationship between genetic alterations and treatment efficacy is essential for optimizing personalized medicine approaches. For example, the case study titled “Impact of Genetic Alterations on Treatment” demonstrates how specific genetic modifications can significantly influence treatment choices, leading to improved outcomes for individuals when therapies are aligned with their molecular profiles.
Moreover, integrating NGS lung cancer findings into clinical decision-making processes is vital for enhancing the precision of treatment for respiratory tumors. As highlighted by Shashank Cingam, MD, in discussions regarding options for individuals with relapsed/refractory multiple myeloma and the application of chimeric antigen receptor (CAR) T-cell therapy, the importance of targeted therapies cannot be overstated. By leveraging expert knowledge and practical examples, healthcare providers can transform treatment strategies for respiratory illnesses, ultimately resulting in better outcomes for individuals and an improved quality of life. As the landscape of lung disease therapy continues to evolve, the influence of NGS lung cancer on treatment strategies remains a critical factor in advancing personalized care.
Navigate Implementation Challenges: Integrating NGS into Clinical Practice
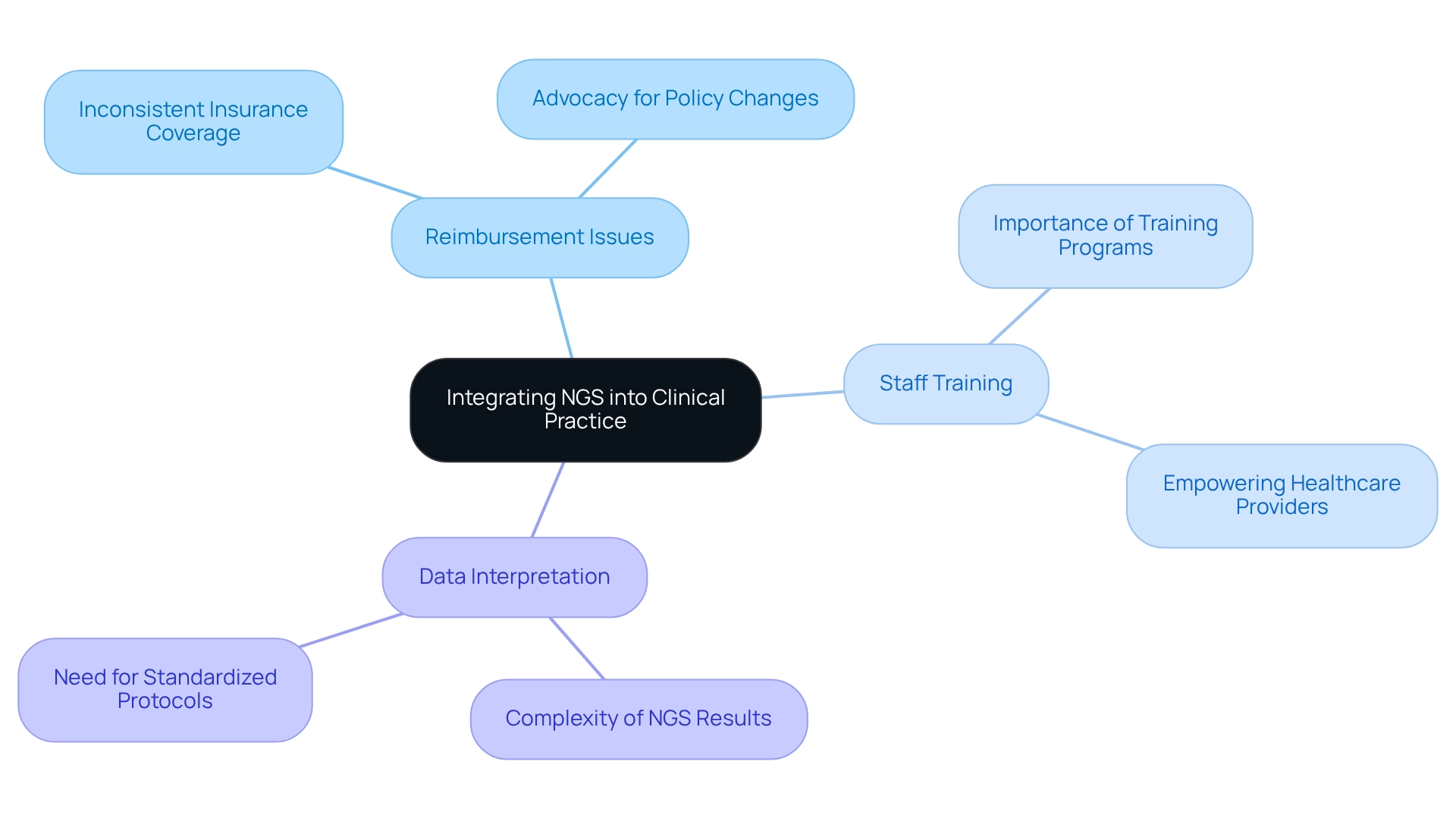
Integrating Next-Generation Sequencing (NGS) into clinical practice presents several significant challenges, particularly regarding reimbursement, staff training, and data interpretation complexities. A primary obstacle is the inconsistency in insurance coverage for NGS testing, which can restrict access to these advanced diagnostic tools. For instance, although the 2018 National Coverage Determination expanded access to NGS for all Medicare beneficiaries with advanced illness, variability in coverage persists, complicating the landscape for healthcare providers and individuals alike.
Moreover, healthcare professionals must be thoroughly equipped to interpret NGS results accurately and comprehend their clinical implications. This necessity underscores the importance of extensive training programs to ensure that staff can effectively utilize NGS data in healthcare settings. Institutions can proactively address these challenges by developing standardized protocols for NGS testing and interpretation, streamlining processes, and enhancing result consistency.
Investing in staff education is crucial, as it empowers healthcare providers to navigate the complexities of NGS data with confidence. Additionally, advocating for policy changes that support reimbursement for NGS testing is essential to facilitate broader adoption. By tackling these barriers head-on, healthcare providers can significantly improve the integration of NGS lung cancer into clinical practice, ultimately enhancing management and patient outcomes.
Conclusion
The integration of Next-Generation Sequencing (NGS) into lung cancer diagnosis and treatment signifies a pivotal advancement in personalized medicine. By enabling the simultaneous analysis of multiple genes, NGS not only enhances diagnostic accuracy but also facilitates early detection of actionable mutations, thereby informing targeted therapy decisions. This technology significantly improves patient stratification for clinical trials and impacts survival rates by tailoring treatment strategies to individual genetic profiles.
However, despite its transformative potential, the implementation of NGS in clinical settings encounters several challenges, including:
- Reimbursement issues
- Staff training needs
- Complexities in data interpretation
Overcoming these barriers is essential to maximize the benefits of NGS, ensuring that patients gain access to the most advanced diagnostic tools available. Investing in education and advocating for supportive policies can empower healthcare providers to fully integrate NGS into their practice, ultimately enhancing patient care and outcomes.
As the landscape of lung cancer management continues to evolve, the role of NGS will only become more critical. By embracing this technology, oncologists can revolutionize treatment strategies, leading to improved efficacy and quality of life for patients. The future of lung cancer care hinges on the successful integration of NGS, marking a significant step towards precision medicine in oncology.


