Overview
The article presents strategies for achieving success in market access within the pharmaceutical industry, underscoring the critical importance of comprehending key concepts such as market access, reimbursement, and stakeholder engagement. It delineates effective strategies, including:
- Early stakeholder engagement
- Demonstrating value
- Tailored pricing
- Leveraging digital tools
- Fostering cross-functional collaboration
These strategies are all underpinned by data insights, enabling stakeholders to navigate the complexities of drug commercialization and enhance patient access to medications. By understanding these elements, industry professionals can better position themselves for success.
Introduction
In the dynamic realm of the pharmaceutical industry, grasping the complexities of market access and drug commercialization is crucial for achieving success. As companies maneuver through a labyrinth of pricing pressures, reimbursement challenges, and regulatory obstacles, the capacity to present a compelling value proposition becomes indispensable.
Projections suggest a notable increase in the market for artificial intelligence in drug discovery, making the integration of advanced analytics not merely advantageous, but essential.
This article explores the fundamental concepts that shape market access strategies, identifies the challenges encountered by pharmaceutical companies, and delineates effective methods to leverage data insights for informed decision-making.
By mastering these components, stakeholders can enhance their positioning in a competitive landscape while ensuring that patients receive timely access to groundbreaking therapies.
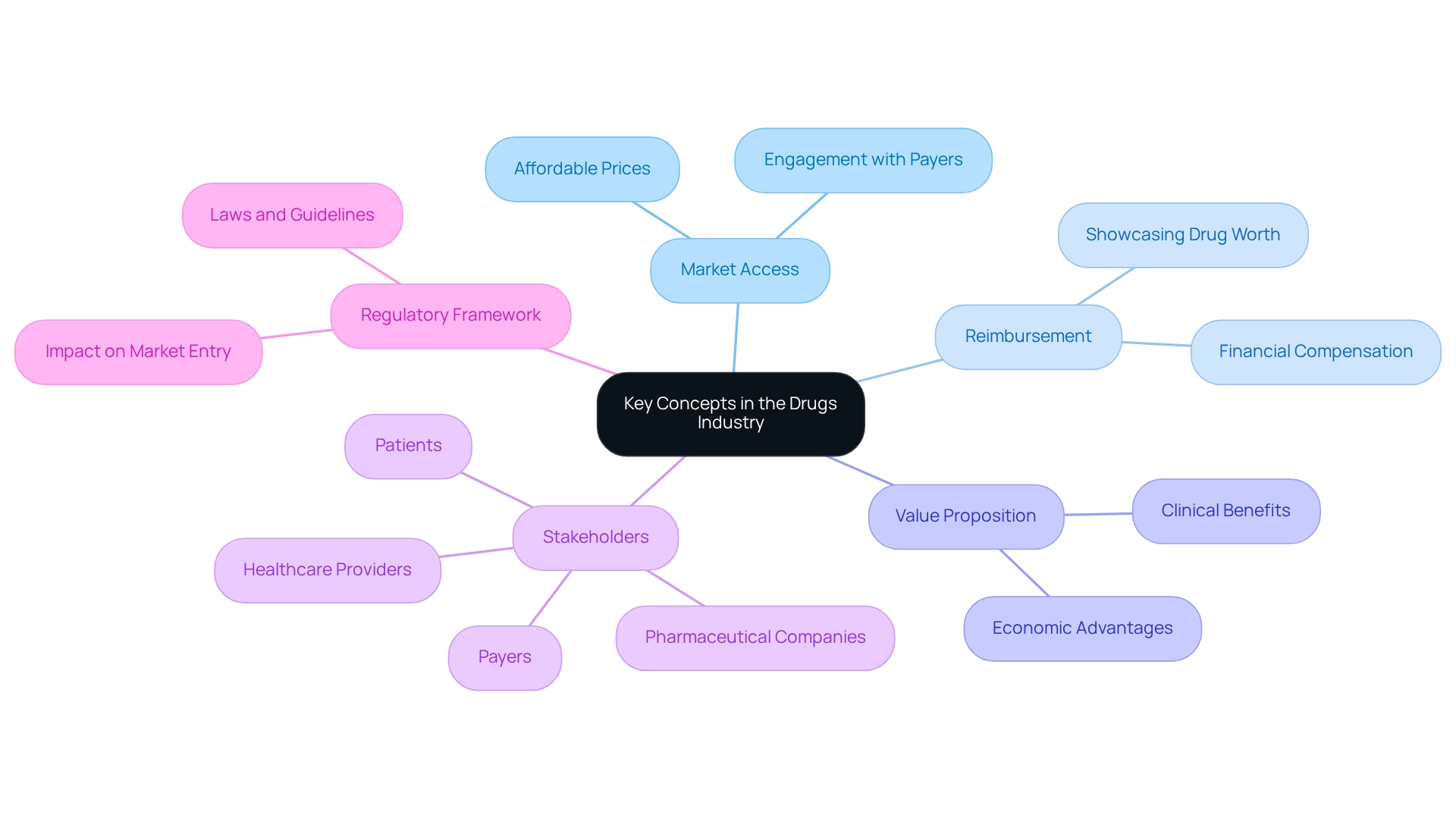
Define Key Concepts in the Drugs Industry
In the pharmaceutical industry, understanding market access and drug commercialization hinges on several key concepts.
Market Access refers to the strategies and processes that ensure patients can obtain medications at affordable prices. It encompasses complex discussions with payers and healthcare providers to enable availability.
Reimbursement is critical as it involves financial compensation for healthcare providers administering drugs. This compensation frequently relies on the ability to showcase the drug’s worth through clinical results and economic advantages, making effective reimbursement plans crucial for commercialization success.
Value Proposition articulates a drug’s unique value to patients and healthcare systems through its clinical and economic benefits. A compelling value proposition is vital for gaining acceptance from stakeholders and payers.
Stakeholders within the healthcare ecosystem include pharmaceutical companies, healthcare providers, payers, and patients. Each stakeholder possesses unique interests and influences that can affect entry strategies.
Regulatory Framework encompasses the laws and guidelines governing drug approval and entry into commerce, which vary by nation and significantly impact market entry. Understanding these regulations is essential for navigating the complexities of drug commercialization.
Current statistics indicate that the global access landscape is evolving rapidly, with projections suggesting that the field of artificial intelligence in drug discovery will reach significant valuations, anticipated to be in the billions of U.S. dollars by 2032. This underscores the importance of leveraging advanced analytics, such as CareSet’s comprehensive Medicare data insights, to optimize production and distribution plans.
Moreover, the development of new medicines in the drugs industry is often uncertain, costly, and characterized by significant failure rates, presenting challenges in drug commercialization. Expert opinions emphasize that effective reimbursement models are foundational to successful commercialization within the drugs industry. For example, case studies show that firms employing artificial intelligence can improve analysis, tailor marketing initiatives, and optimize pricing approaches, ultimately boosting engagement and negotiation stances.
One significant case study titled “AI’s Role in Pharmaceutical Commercialization” demonstrates how AI is transforming pharmaceutical commercialization through improved analysis and pricing strategies.
Furthermore, as Roman Bevz, Principal Domain Consultant in Life Sciences, pointed out, “Interestingly, this technology can also be utilized to propose therapies or health plans related to cultural norms and religion-based dietary constraints,” emphasizing the significance of addressing varied patient requirements in strategic planning.
In conclusion, mastering these key concepts is vital for navigating the drugs industry and pharmaceutical environment in 2025 and beyond. This mastery ensures that stakeholders can effectively tackle the challenges of entry and reimbursement, empowered by innovative solutions like those provided by CareSet.
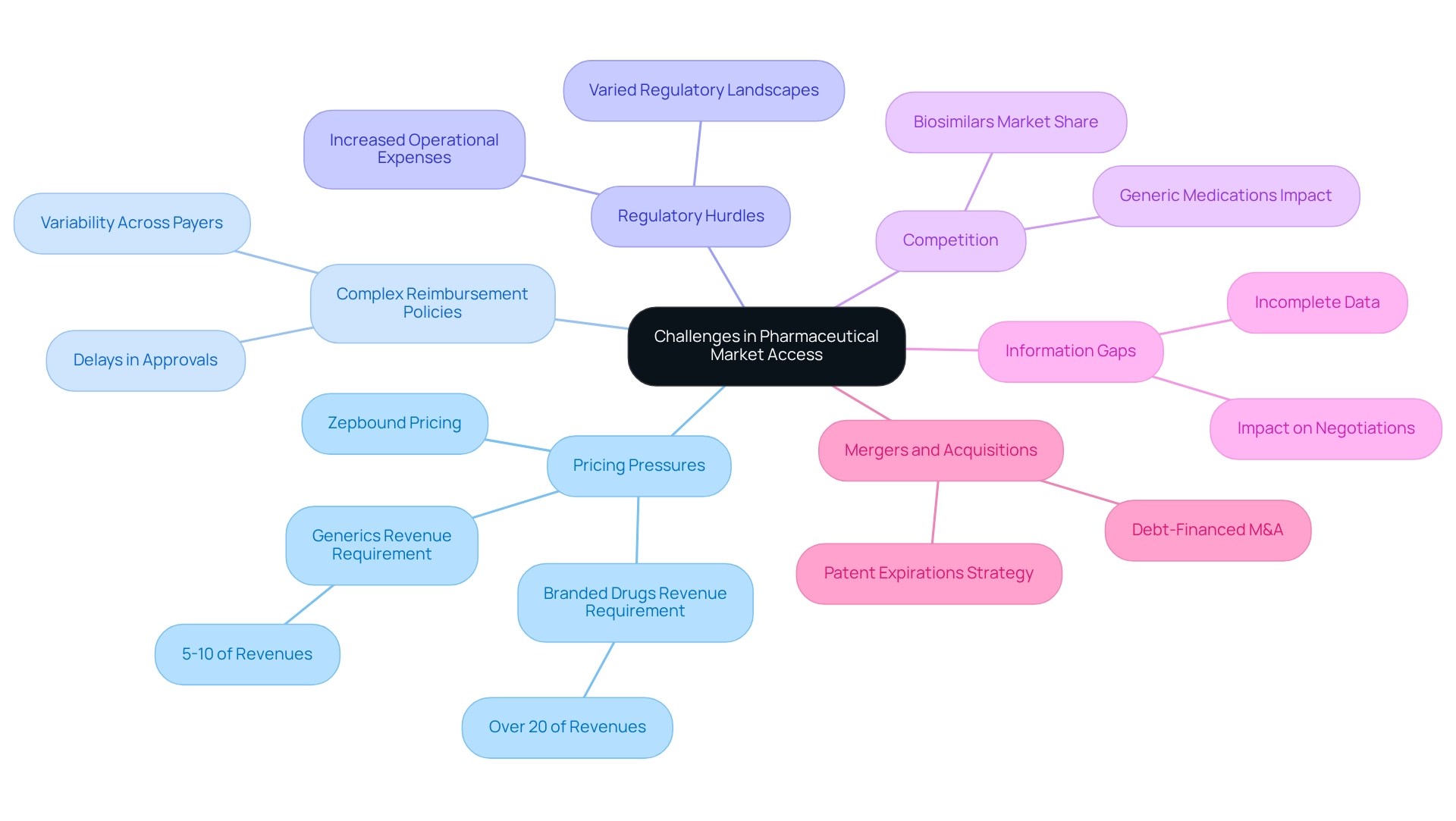
Identify Challenges in Pharmaceutical Market Access
In the drugs industry, pharmaceutical firms face numerous challenges in obtaining market entry, increasingly influenced by growing pricing pressures and complex reimbursement environments.
By 2025, the drugs industry is grappling with significant pricing scrutiny from payers and regulators due to escalating pricing pressures. Companies are compelled to heavily reinvest to sustain revenue growth, with branded drugs requiring over 20% of revenues and generics needing at least 5%-10%. This financial strain constrains the ability to set profitable prices in the drugs industry, as evidenced by the rising costs of medications, such as the Zepbound single-dose vials priced at $499. This example underscores the broader trend of increasing medication costs in the drugs industry, reflecting the challenges companies face in justifying pricing amidst heightened scrutiny. CareSet’s extensive healthcare data insights can empower companies to assess pricing approaches effectively and validate their pricing models through robust data.
-
Complex Reimbursement Policies: The variability in reimbursement processes across different payers complicates strategies for pharmaceutical firms. They must navigate a patchwork of regulations that can delay reimbursement approvals and affect overall access. By leveraging CareSet’s information solutions, companies can gain insights into reimbursement trends and optimize their strategies accordingly.
-
Regulatory Hurdles: The varied regulatory landscapes across nations present further challenges for the drugs industry, frequently causing delays in market entry and increasing operational expenses. Companies must remain agile to adapt to these differing requirements. CareSet’s data insights can assist firms in staying informed about regulatory changes and streamlining their compliance processes.
Competition within the drugs industry: The presence of generic medications and biosimilars continues to diminish the market share and pricing influence of branded products. This competitive environment necessitates innovative strategies to maintain a strong presence. CareSet’s analytics can provide competitive insights that help companies identify opportunities and threats in their sector.
- Information Gaps: Incomplete or flawed information can severely hinder the ability to effectively demonstrate a drug’s value. This impacts negotiations with payers, as robust evidence is crucial for justifying pricing and securing favorable reimbursement terms. For instance, a lack of thorough information can complicate efforts to demonstrate treatment effectiveness, further obstructing market entry. CareSet’s solutions can bridge these data gaps, enabling companies to present compelling evidence of their products’ value.
The ongoing trend of mergers and acquisitions (M&A) within the drugs industry underscores the urgency for companies to address these challenges. As firms seek to sustain revenue growth amid patent expirations, reliance on debt-financed M&A is expected to persist, potentially increasing debt leverage and applying pressure on profit margins. These M&A strategies are frequently employed in the drugs industry to enhance product portfolios and improve market entry capabilities in response to competitive pressures.
Expert insights highlight the critical balance between affordability and medical advancement within the drugs industry, emphasizing the need for pharmaceutical companies to navigate these complexities strategically. As Ben Link noted, “This medication increased by 25.7%, which is not a huge surprise given the prevalence of global diabetes and its continued increase.” This statement underscores the wider trends in healthcare that complicate entry efforts, particularly as companies endeavor to showcase the value of their products in a challenging pricing landscape.
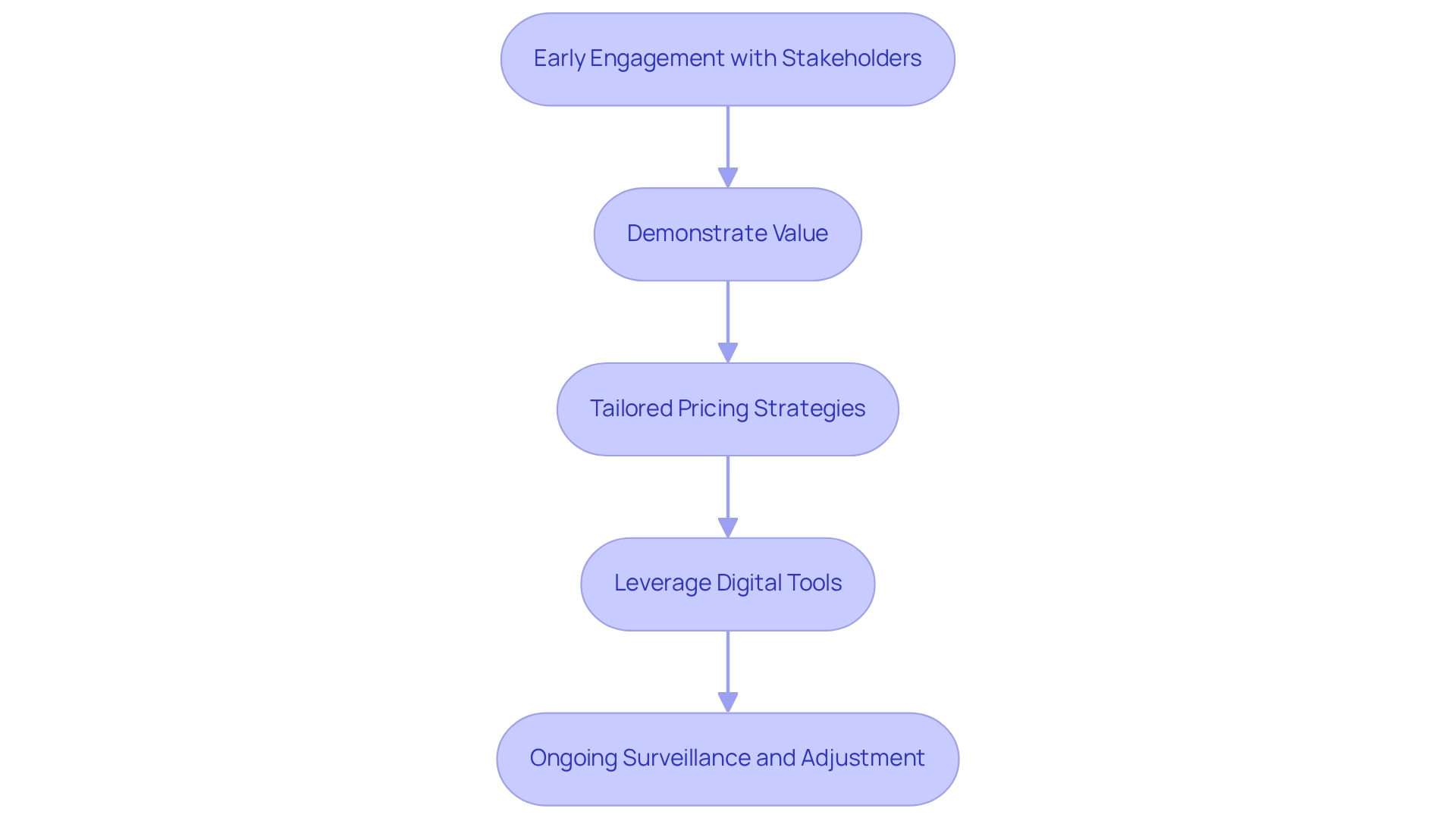
Outline Effective Market Access Strategies
To enhance market access, pharmaceutical companies can adopt several effective strategies:
-
Early Engagement with Stakeholders: Involving key stakeholders early in the drug development process is crucial for aligning expectations and requirements. Statistics indicate that early engagement can lead to a 28% improvement in predicting real-world patient responses, underscoring its importance in shaping successful outcomes. As Steven Zona notes, “Game-changing innovation at scale is the daily routine for Market Access professionals at Johnson & Johnson—but it might surprise you that storytelling plays a role in how we do it.” This highlights the significance of narrative in engaging stakeholders effectively.
-
Demonstrate Value: Clearly articulating the clinical and economic value of a drug through robust evidence and real-world data is essential. CareSet’s monthly Medicare updates provide valuable insights into drug utilization and treatment pathways, answering critical questions such as which diseases providers diagnose and treat, and how patients navigate from diagnosis to treatment. This allows companies to establish trust with stakeholders and assist in pricing negotiations and competitive positioning.
-
Tailored Pricing Strategies: Developing pricing models that reflect the drug’s value while considering market dynamics and payer expectations is vital. Companies that implement tailored pricing strategies can better navigate the complexities of reimbursement and access, especially when informed by CareSet’s insights into Medicare Part D Plans and treatment approvals.
-
Leverage Digital Tools: Utilizing digital platforms for information collection and stakeholder engagement streamlines processes and enhances communication. CareSet’s comprehensive healthcare information insights enable pharmaceutical companies to make informed decisions rapidly, resulting in a 29% quicker decision-making process across clinical and commercial operations, as reported by Accenture’s Healthcare Analytics Benchmark Survey.
-
Ongoing Surveillance and Adjustment: Frequently evaluating industry conditions and modifying approaches accordingly ensures that firms stay competitive and responsive to changes. CareSet’s insights from over 62 million beneficiaries and 6 million providers enable a proactive approach in a rapidly evolving healthcare landscape.
Through the adoption of these approaches, backed by CareSet’s cutting-edge Medicare information solutions, pharmaceutical firms can successfully maneuver access obstacles and improve their overall influence in the industry.
Leverage Data Insights for Strategic Decision-Making
Utilizing insights can significantly improve decision-making in the pharmaceutical sector. By employing comprehensive information, including insights from CareSet Systems’ innovative data science products such as predictive analytics and real-world evidence tools, companies can identify trends in prescribing behaviors and patient demographics, which informs targeted promotional approaches. Current analytics trends indicate a growing emphasis on understanding patient journeys to optimize outreach efforts. Looking ahead to 2025, there is expected to be a heightened focus on incorporating social determinants of health into analytics to better customize strategies for various patient populations.
Predictive modeling through predictive analytics, backed by CareSet’s Medicare insights, enables organizations to project industry trends and foresee patient needs, promoting proactive modifications to strategies. This approach is increasingly vital as the drugs industry shifts towards personalized medicine, with AI integration in treatments showing an 18% improvement in survival rates. Notably, this technology can also suggest treatments or health plans that consider cultural norms and religion-based dietary restrictions, as highlighted by Roman Bevz, Principal Domain Consultant in Life Sciences.
Gathering and analyzing real-world evidence, including comprehensive Medicare data solutions from CareSet, is crucial for demonstrating a drug’s effectiveness and safety within the drugs industry across diverse patient populations. This evidence supports strong value propositions, essential for access and reimbursement discussions. A pertinent case study is the creation of new molecular entities in the U.S., which emphasizes the nation’s vital role in the drugs industry despite challenges in the journey from discovery to sales.
Observing competitor activities and dynamics within the drugs industry allows pharmaceutical companies to recognize opportunities and threats quickly. This agility is vital in a swiftly changing environment, where prompt insights can determine positioning within the industry.
Gathering insights from healthcare providers and patients is crucial for improving product offerings and boosting engagement approaches. Involving stakeholders guarantees that products fulfill the genuine requirements of the drugs industry, cultivating stronger connections and improved results.
Looking forward, the future of AI in the drugs industry encompasses increased application in digital health, real-time analytics, and global pandemic readiness, which will further influence the industry’s entry plans. Integrating these approaches, especially those guided by CareSet’s data-driven insights, not only enhances decision-making but also positions companies in the drugs industry to navigate the intricacies of the pharmaceutical environment effectively, ultimately leading to better patient care and commercial success. Note: CareSet Systems announced these innovative information science products on October 19, 2016.

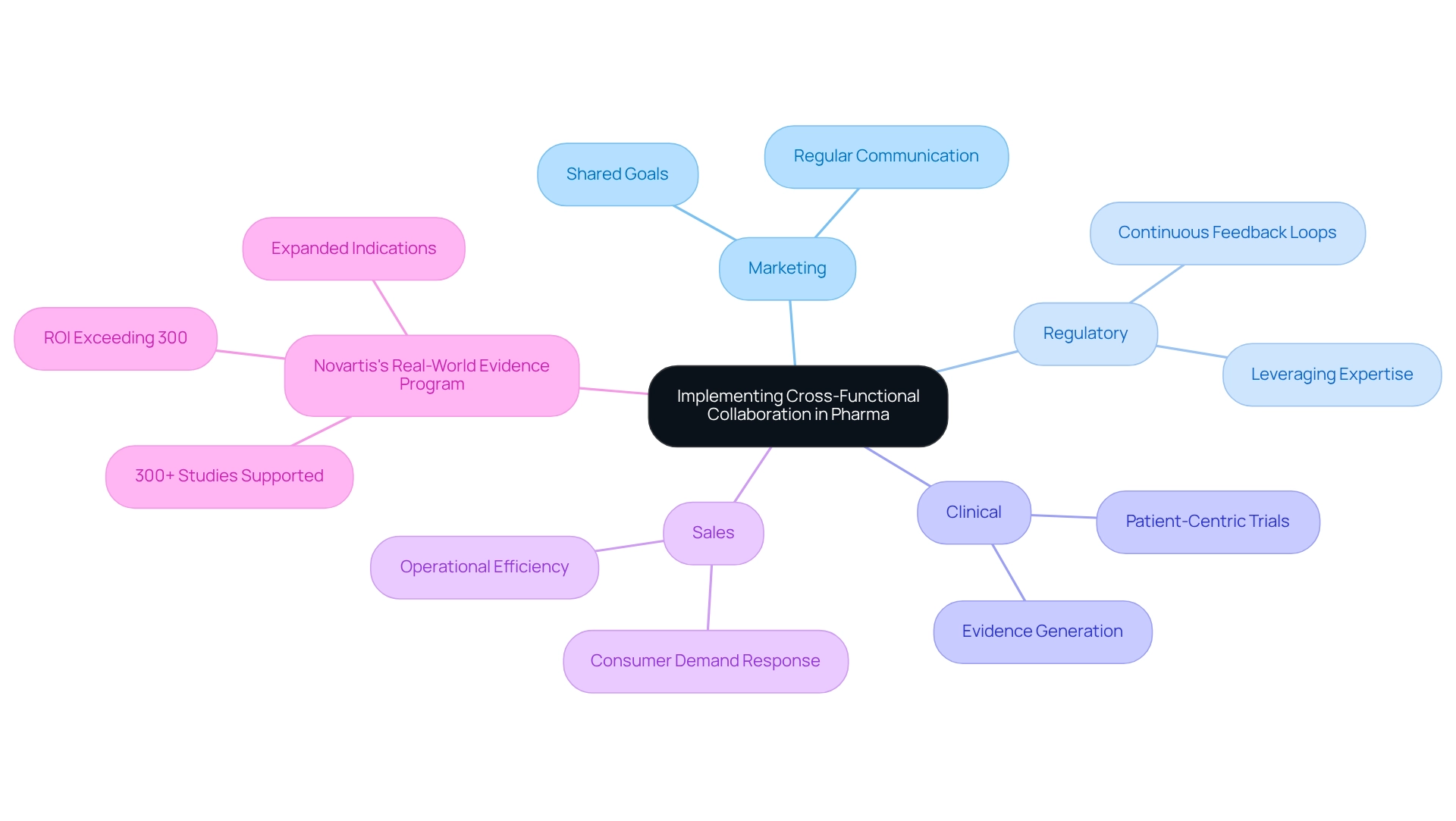
Implement Cross-Functional Collaboration
To ensure a successful market access strategy, pharmaceutical companies must prioritize cross-functional collaboration. Integrating teams is essential; fostering collaboration among marketing, regulatory, clinical, and sales teams aligns objectives and messaging effectively. Leveraging CareSet’s comprehensive Medicare data insights informs decision-making, creating a robust foundation for strategy. Establishing shared goals cultivates a sense of collective purpose, driving all departments toward unified objectives. Regular communication is vital—scheduling consistent meetings to discuss progress, share insights, and collaboratively address challenges utilizes data from CareSet to guide discussions. Furthermore, leveraging diverse expertise enhances overall strategy and execution, supported by actionable insights from CareSet’s data solutions. Continuous feedback loops between teams refine strategies and improve outcomes.
The impact of integrated teams on market access strategies is significant. For instance, Novartis’s Real-World Evidence Program illustrates how cross-functional teamwork can enhance entry into the industry. This initiative has supported over 300 studies, generating evidence that not only secured expanded indications for existing products but also informed patient-centric clinical trials. Such collaborative efforts have been associated with an estimated return on investment surpassing 300%, emphasizing the financial advantages of efficient teamwork.
Expert views indicate that integrating teams is crucial for access success. A Whole Brain® approach ensures that the diverse needs of all stakeholders are addressed, enhancing the overall effectiveness of business strategies. In 2025, the focus on cross-functional collaboration continues to be a strategic necessity, particularly as pharmaceutical companies encounter challenges such as information quality issues and regulatory compliance concerns.
By adopting integrated teams and leveraging CareSet’s extensive Medicare data solutions, organizations can manage these complexities and promote successful initiatives. Tackling these challenges through teamwork not only boosts operational efficiency but also increases the capacity to respond to consumer demands effectively.
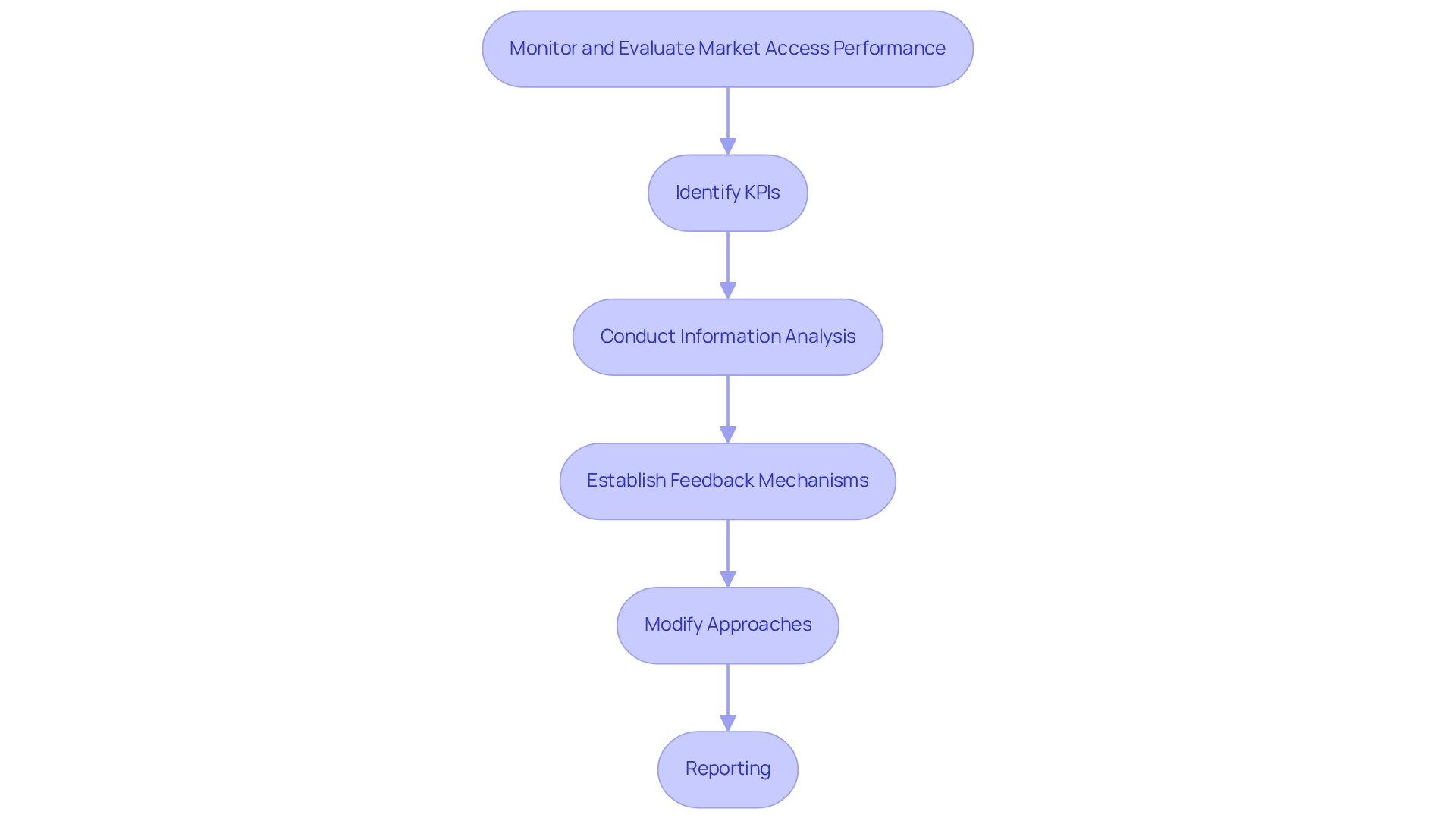
Monitor and Evaluate Market Access Performance
To ensure the efficiency of commercial entry plans, companies must consistently observe and assess their performance through a systematic method.
Key Performance Indicators (KPIs) are essential for gauging success. Significant metrics encompass share, patient entry rates, and reimbursement success, which collectively offer a clear view of performance in the industry. KPIs act as a guide directing strategic choices concerning marketing and customer relationship management.
Information Analysis is vital for evaluating the effect of market entry approaches on sales and patient results. By incorporating insights from more than 100 external information sources, including CareSet’s extensive Medicare solutions, companies can achieve a deeper understanding of treatment patterns and patient demographics. This facilitates informed decision-making that corresponds with the requirements of over 62 million beneficiaries and 6 million providers, ultimately improving strategic healthcare choices.
Feedback Mechanisms play a crucial role in establishing feedback loops with stakeholders, enabling organizations to collect valuable insights on the effectiveness of their approaches. This continuous conversation aids in recognizing areas for enhancement and promotes a culture of teamwork.
Modify Approaches: Businesses should remain flexible and prepared to adjust their strategies based on performance data and changing industry conditions. This adaptability is key to optimizing outcomes and ensuring sustained success.
Reporting: Regular reporting of findings to stakeholders is vital for maintaining transparency. This practice not only builds trust but also encourages a culture of continuous improvement, where insights lead to actionable changes. By effectively conveying outcomes, organizations can enhance stakeholder involvement and foster a cooperative atmosphere.
A pertinent case study, ‘Improving Predictions through Market Access Tracking,’ demonstrates how companies can utilize data from public resources and previous plans to make informed forecasts regarding upcoming product launches. This method enables organizations to gain insights from past experiences and adjust their plans to match evolving conditions, resulting in more precise forecasts and improved decision-making.
In 2025, the focus on observing performance related to entry in the drugs industry will be more essential than ever, as the pharmaceutical environment continues to change. By concentrating on robust KPIs and data-driven strategies, including insights from CareSet, organizations can enhance their market access efforts and ultimately improve patient outcomes.
Conclusion
Navigating the complexities of market access and drug commercialization in the pharmaceutical industry is essential for success in an increasingly competitive landscape. Understanding key concepts such as market access, reimbursement, and the value proposition lays the foundation for effective strategies. Stakeholders must engage early, demonstrate value through real-world evidence, and tailor pricing strategies to align with the evolving healthcare environment.
Pharmaceutical companies face significant challenges, including rising pricing pressures, complex reimbursement policies, and regulatory hurdles. Leveraging data insights is vital for informed decision-making, enabling organizations to anticipate market trends, optimize strategies, and enhance patient access to innovative therapies. Cross-functional collaboration further strengthens market access efforts, as integrating diverse expertise leads to more effective and agile responses to industry demands.
Ultimately, the future of pharmaceutical market access hinges on the ability to adapt and innovate. By continuously monitoring performance and embracing data-driven approaches, companies can not only overcome challenges but also significantly improve patient care and achieve lasting success in the dynamic healthcare landscape. The integration of advanced analytics and stakeholder engagement will be pivotal in ensuring that groundbreaking therapies reach those who need them most, marking a new era of pharmaceutical excellence.


