Overview
Mastering marketing within the pharmaceutical industry for effective market access requires a nuanced understanding of regulatory requirements, collaboration with stakeholders, adaptation to evolving reimbursement policies, and the strategic use of data analytics. A strategic approach in these areas—such as the establishment of dedicated regulatory teams, early engagement with healthcare professionals, vigilant monitoring of reimbursement trends, and the application of advanced analytics—significantly enhances market access and ultimately leads to improved health outcomes.
By implementing these strategies, organizations can navigate the complexities of the market more effectively, ensuring they remain competitive and responsive to industry demands.
Introduction
In the dynamic world of pharmaceuticals, companies encounter a complex array of challenges as they seek market access and patient engagement. Navigating intricate regulatory landscapes and adapting to fluctuating reimbursement policies are just a few of the hurdles that must be overcome.
Effectively engaging stakeholders and leveraging data analytics have become essential strategies that can profoundly impact product accessibility and outcomes. With the right insights and proactive approaches, pharmaceutical organizations can not only comply with regulations but also enhance their market strategies, ultimately leading to improved patient care.
This article explores key strategies that empower companies to navigate these complexities and thrive in a competitive environment.
Navigate Regulatory Requirements for Market Access
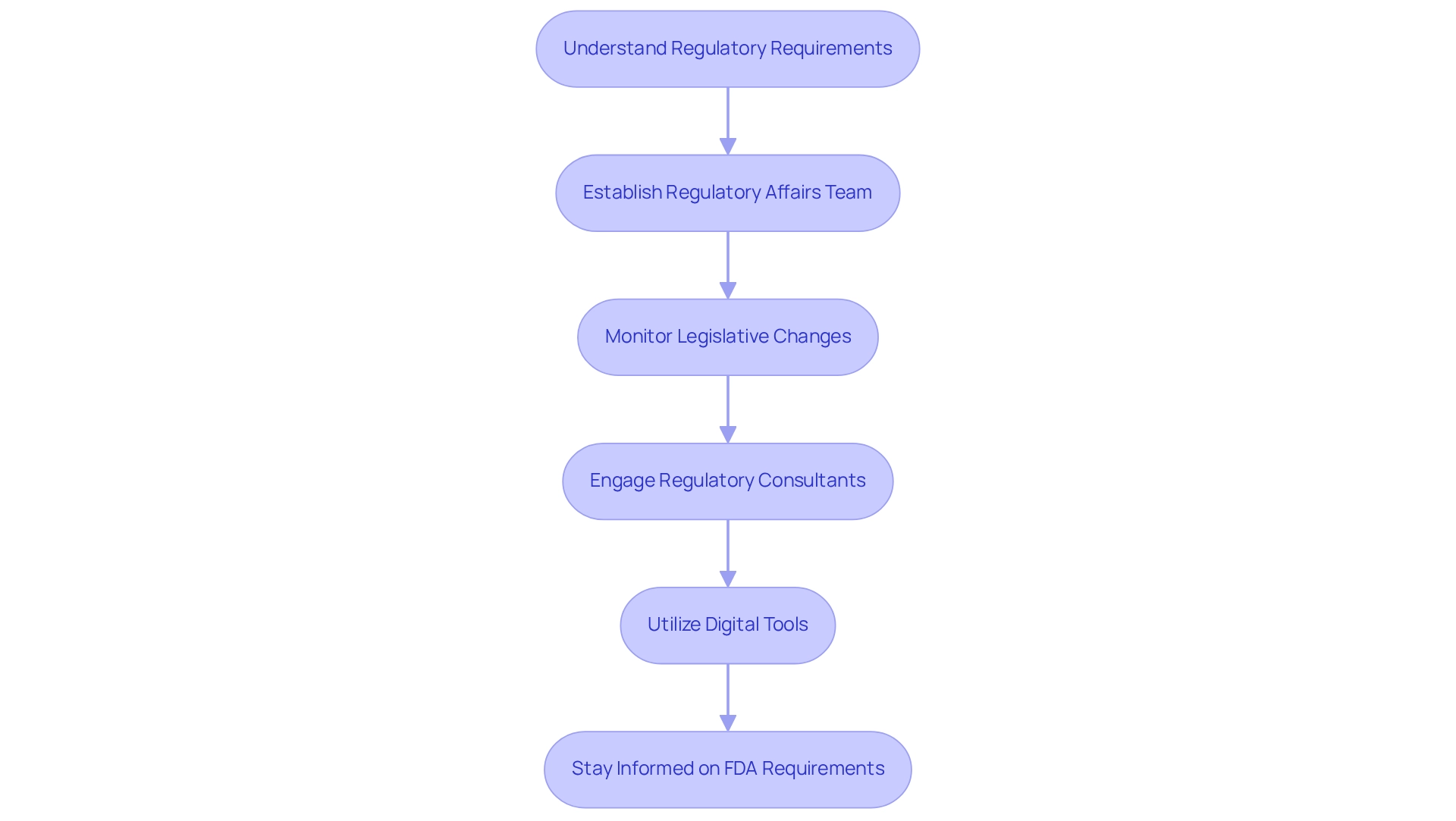
Navigating regulatory requirements is essential for pharmaceutical companies aiming for successful industry entry. A comprehensive understanding of the specific rules relevant to their offerings across various sectors is crucial. This encompasses a thorough familiarity with FDA guidelines and state-specific regulations.
Establishing a dedicated regulatory affairs team is imperative; this team must continuously monitor legislative changes and ensure compliance throughout the product lifecycle. Engaging regulatory consultants can provide invaluable insights and streamline the approval process. Companies that proactively engage with the FDA during the development phase often reap the benefits of smoother approvals and reduced time-to-market.
Furthermore, utilizing digital tools that incorporate over 100 external information sources to monitor regulatory changes allows companies to stay ahead of compliance obligations, thereby enhancing their market access strategies. CareSet’s comprehensive healthcare data insights significantly aid this process, offering valuable information that empowers pharmaceutical companies to navigate complex regulatory landscapes effectively.
For instance, Akitra provides ready-to-use templates for new policies and procedures, facilitating compliance adoption for organizations. This proactive strategy not only mitigates risks but also fosters long-term strategic growth, as evidenced by CareSet’s thorough method that enhances outcomes for individuals and optimizes the lifecycle management of pharmaceutical products.
Additionally, as highlighted by Anoop VK, AVP of Cloud Engineering & CISO, the precision in control definitions and continuous monitoring offered by tools like Akitra ensure early risk identification, making them invaluable assets in compliance journeys. Ultimately, staying informed about the 2025 FDA regulatory requirements for pharmaceutical market access is essential for companies to maintain competitiveness and compliance.
Collaborate with Stakeholders to Enhance Product Accessibility
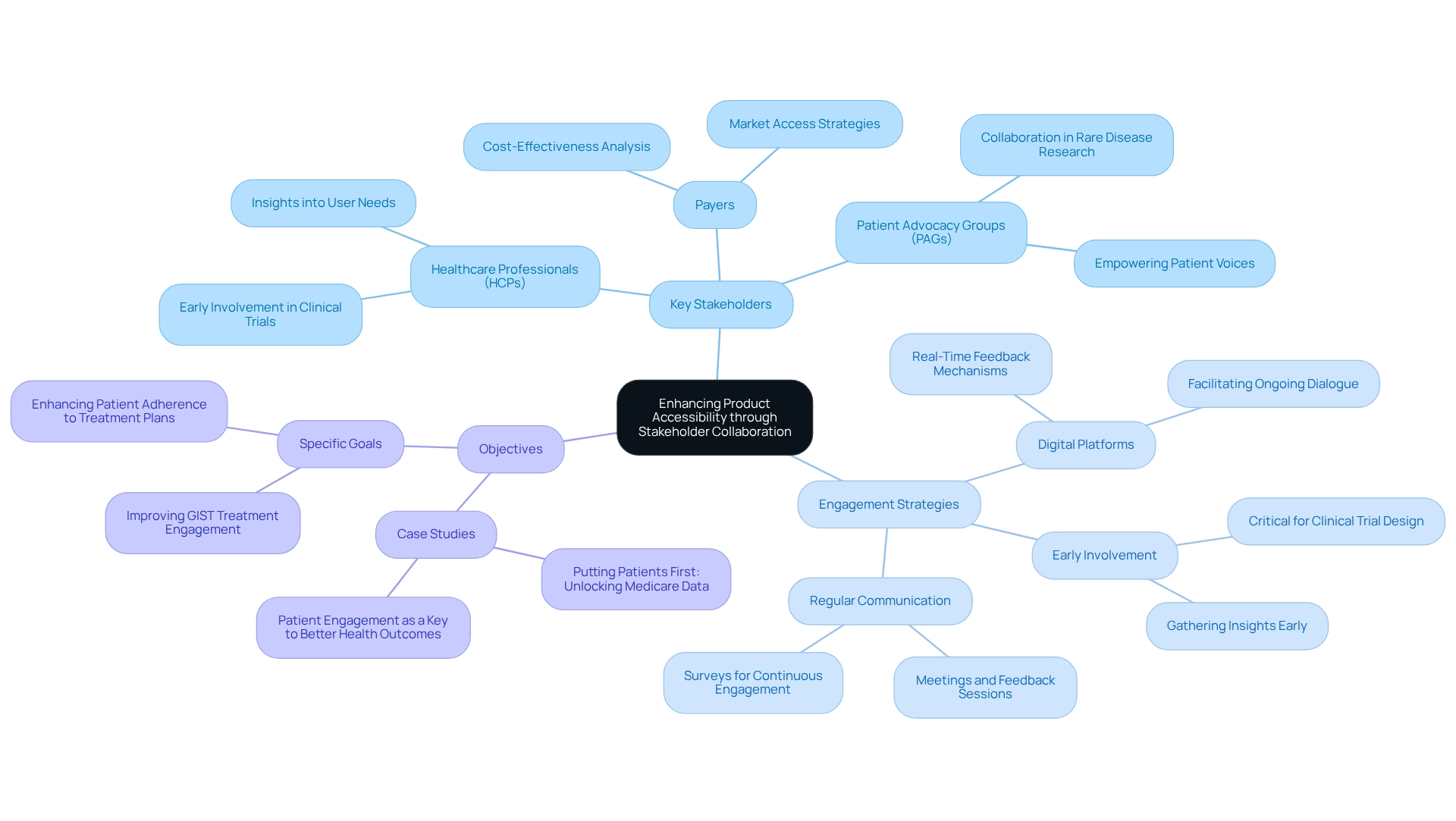
Successful cooperation in pharmaceutical industry marketing hinges on the identification and involvement of key stakeholders, including healthcare professionals (HCPs), payers, and advocacy groups (PAGs). Engaging these stakeholders early in the product development process is essential for gathering insights into user needs and identifying treatment gaps. Regular communication through meetings, surveys, and feedback sessions cultivates strong relationships and ensures stakeholders feel valued.
Involving HCPs early is a critical strategy. Companies that engage HCPs in clinical trial design often gain invaluable insights that enhance the relevance and effectiveness of their studies. This approach not only elevates the quality of clinical trials but also contributes to improved health outcomes.
Focusing on specific objectives is equally important. For example, the case study titled “Putting Patients First: Unlocking Medicare Data to Empower HCP” underscores the business objective of increasing timely engagement with physicians regarding the fourth line of therapy for Gastrointestinal Stromal Tumor (GIST), significantly impacting patient care.
Leveraging digital platforms for stakeholder engagement facilitates ongoing dialogue and provides real-time feedback, crucial for adapting marketing strategies to meet evolving industry demands. Furthermore, CareSet utilizes over 100 external data sources, incorporating insights from more than 62 million beneficiaries and 6 million providers. This highlights the importance of data-informed strategies in stakeholder engagement and development.
Statistics indicate that effective stakeholder involvement can profoundly influence development timelines and market access strategies. By prioritizing cooperation with stakeholders, pharmaceutical industry marketing can enhance the accessibility of offerings and ultimately lead to better health outcomes.
As Christine Jacob aptly noted, “And in general, the patient engagement occurs a little too late in the process,” emphasizing the critical need for early stakeholder involvement in the development journey. Additionally, pharmaceutical companies engaged in rare disease research should collaborate with agencies experienced in working with PAGs, further underscoring the significance of these partnerships in specialized research areas.
Adapt Strategies to Changing Reimbursement Policies
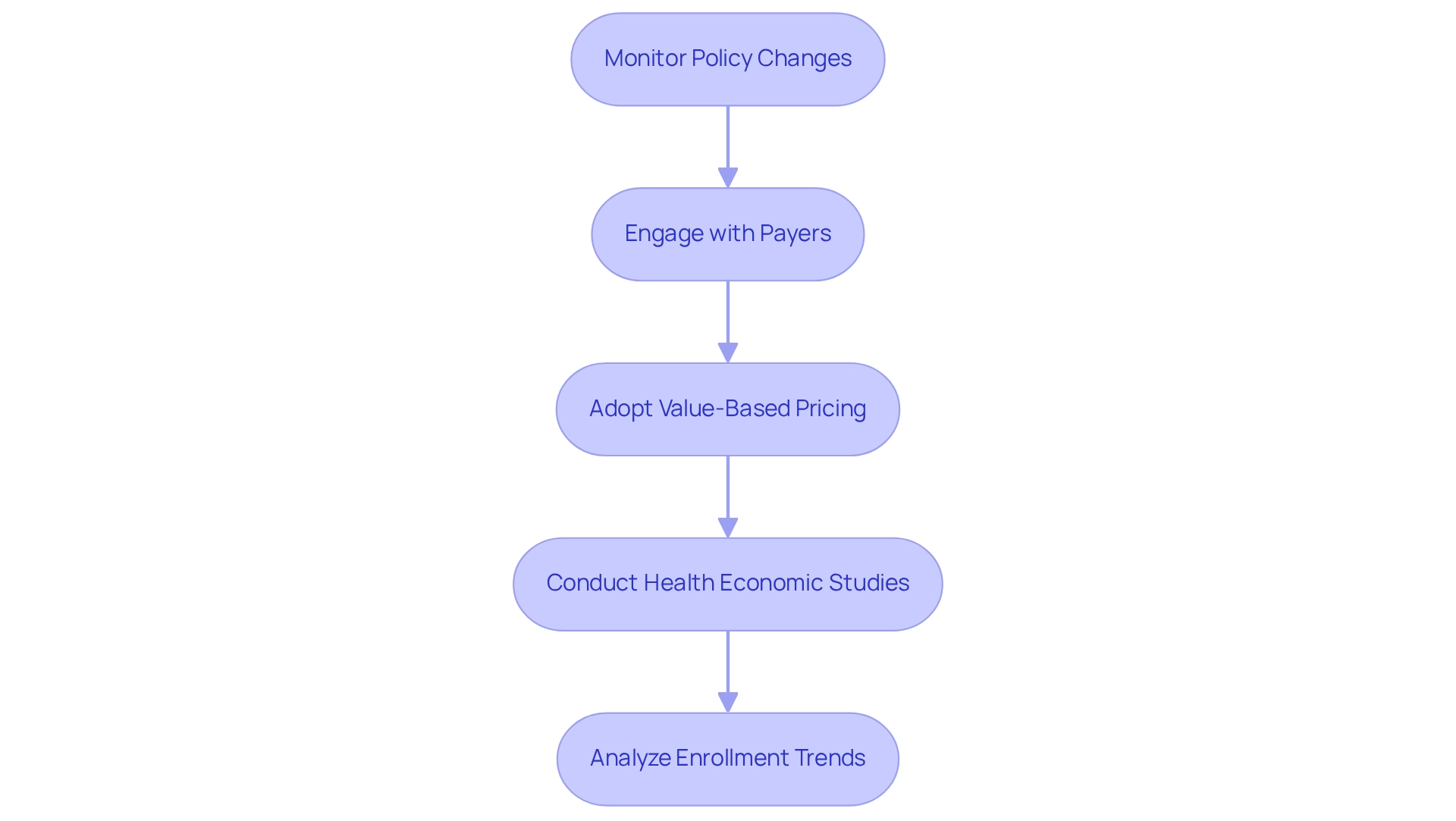
To effectively navigate the shifting landscape of reimbursement policies, pharmaceutical companies must develop a comprehensive market access strategy that integrates pharmaceutical industry marketing while prioritizing continuous monitoring of policy changes at both federal and state levels. This strategy necessitates a thorough analysis of how fluctuations in reimbursement rates, coverage decisions, and formulary placements impact accessibility to products.
Engaging with payers early in the offering lifecycle is crucial for negotiating favorable reimbursement terms. For example, adopting value-based pricing models can align costs with patient outcomes, thereby enhancing appeal to payers. However, the complexity of patient access may arise due to potential shifts in formulary coverage and availability.
Furthermore, conducting health economic studies that illustrate a product’s cost-effectiveness can significantly bolster reimbursement negotiations. CareSet’s comprehensive healthcare data insights empower pharmaceutical companies by enhancing their marketing strategies through essential information on industry trends and payer behaviors, enabling more informed decision-making.
The anticipated decline in fully insured group enrollment from 53 million in 2023 to 49 million in 2028 underscores the significance of ongoing observation of policy changes and their potential effects on access strategies. By adopting a proactive and flexible approach in pharmaceutical industry marketing, companies can mitigate risks associated with reimbursement changes and ensure continuous access to the market, ultimately improving care and outcomes for individuals.
Notably, 79% of providers are focusing on controlling costs and adopting a cautious approach, reflecting the current industry sentiment. Additionally, the evolving regulations affecting Pharmacy Benefit Managers (PBMs) illustrate how regulatory changes are prompting adaptations in reimbursement models.
Overall, grasping the implications of healthcare reimbursement trends on care and outcomes is essential for developing effective strategies.
Leverage Data Analytics for Informed Marketing Decisions
Pharmaceutical industry marketing necessitates that firms leverage analytics to uncover insights into market trends, consumer demographics, and prescribing behaviors. By utilizing advanced analytics tools, such as those provided by CareSet Systems, organizations can effectively segment their target audiences and customize marketing messages to resonate with specific healthcare providers. For instance, predictive analytics can pinpoint which providers are most likely to prescribe a new medication, thereby enabling targeted outreach efforts that maximize impact.
Moreover, CareSet’s innovative Medicare solutions significantly enhance the analysis of real-world information, illuminating treatment patterns and outcomes that are vital for informing product positioning and messaging strategies. CareSet’s monthly Medicare updates address critical business inquiries, such as the diseases providers diagnose and treat, how patients navigate their treatment journeys, and the treatments approved by Medicare Part D Plans. Organizations prioritizing information analytics not only improve their marketing effectiveness but also enhance their agility in responding to market fluctuations. Notably, from the dawn of civilization to 2003, 5 exabytes of information were generated; now, that volume is produced every two days, highlighting the urgent need for advanced analytics tools in this swiftly evolving landscape.
Establishing a data-driven culture within the organization empowers teams to make informed decisions that align with overarching business objectives. As emphasized in the case study ‘Strategies for Sustainable Data-Driven Growth,’ organizations aiming for a sustainable competitive advantage through analytics should invest in information infrastructure and cultivate multidisciplinary teams. This approach can transform healthcare information into valuable insights, thereby improving patient outcomes and operational efficiency.
As the pharmaceutical environment evolves, the utilization of predictive analytics, particularly through CareSet’s comprehensive insights and patient journey mapping, will be essential for refining marketing strategies and achieving sustainable growth. The rapid evolution of smart clinical spaces, described as ‘Ambient Intelligence,’ underscores the importance of unobtrusively collecting and analyzing data to enhance decision-making processes.

Conclusion
Navigating the complexities of market access in the pharmaceutical industry necessitates a multifaceted approach that encompasses regulatory requirements, stakeholder collaboration, and reimbursement strategies. By establishing dedicated regulatory teams and leveraging digital tools, companies can ensure compliance and streamline their market entry processes. Engaging healthcare professionals and patient advocacy groups early in product development fosters valuable insights that enhance accessibility and improve patient outcomes.
Moreover, adapting to evolving reimbursement policies is crucial for maintaining product accessibility. Companies must continuously monitor policy changes and engage payers to negotiate favorable terms that align product costs with patient outcomes. The integration of comprehensive data analytics further empowers pharmaceutical organizations, enabling them to make informed marketing decisions and tailor strategies to meet the needs of diverse patient populations.
Ultimately, the intersection of regulatory navigation, stakeholder engagement, reimbursement adaptability, and data-driven marketing establishes a robust framework for pharmaceutical companies aspiring to thrive in a competitive landscape. By embracing these strategies, organizations can not only enhance their market access but also significantly contribute to improved patient care and health outcomes. The proactive and informed approaches discussed are essential for future success, underscoring the critical importance of strategic planning in the ever-evolving pharmaceutical sector.

