Overview
Mastering market access in the pharmaceutical industry is essential for ensuring that innovative therapies are available and reimbursed within healthcare systems. This not only impacts patient outcomes but also shapes the future of healthcare delivery. Effective market entry strategies, supported by robust data analytics and stakeholder engagement, are crucial for navigating regulatory challenges. In a rapidly evolving landscape, these strategies are key to achieving commercial success and ensuring that patients benefit from the latest advancements in medical science.
Introduction
In the rapidly evolving pharmaceutical landscape, market access has emerged as a critical factor determining the success of new therapies. This multifaceted concept encompasses a complex web of strategies and negotiations designed to ensure that innovative medications reach patients in a timely and cost-effective manner. As the industry grapples with unique challenges posed by the introduction of advanced biologics and monoclonal antibodies, the need for robust market access strategies becomes even more pronounced.
By harnessing comprehensive data analytics and fostering strong relationships with key stakeholders, pharmaceutical companies can effectively navigate regulatory hurdles and enhance their market positioning. This article delves into the intricate nature of market access, underscoring its significance, the challenges encountered, and the strategic approaches that can drive successful product launches and improve patient outcomes.
Define Market Access and Its Importance in Pharmaceuticals
Market entry strategies, policies, and negotiations are essential for ensuring market access in the pharmaceutical industry, as well as the availability and reimbursement of pharmaceutical products within healthcare systems. Its importance is paramount, as it guarantees timely and affordable access to medications for patients who can benefit from them. Effective entry strategies are pivotal in determining a drug’s commercial success, influencing how well a product is integrated into healthcare systems and perceived by key stakeholders, including payers, healthcare providers, and patients.
As we look towards 2025, the landscape of commercial entry is undergoing significant transformation, particularly with the emergence of new generation biologics and monoclonal antibodies (mAbs). These innovations present unique challenges that demand innovative solutions. CareSet’s analysis of over $1.1 trillion in annual Medicare claim data, coupled with insights from more than 100 external sources, empowers pharmaceutical companies to identify new healthcare provider targets and effectively analyze prescribing patterns. This comprehensive Medicare information solution not only enhances strategic initiatives but also addresses the specific challenges posed by these advanced therapies, ensuring that entry strategies are tailored to meet their unique needs.
Successful market entry examples underscore the critical role of robust data in navigating reimbursement challenges. By mapping patient treatment pathways and understanding how providers transition patients from diagnosis through various lines of therapy, companies can tailor their approaches to meet the specific demands of healthcare systems and patients alike. Furthermore, the launch of CareSet’s new data science products amplifies the capacity to analyze treatment approvals and prescribing trends, offering actionable insights derived from Medicare claims data. As industry experts, including Tom Bollyky, highlight, “The Health Impact Fund plan is both innovative and timely… a market-based approach… should be well-received by potential donors and industry participants alike.” This emphasizes the necessity of integrating actionable intelligence into distribution strategies, significantly impacting drug commercial success and ensuring that innovative therapies reach the patients who need them most.
Ultimately, mastering market access in the pharmaceutical industry is crucial for companies striving to thrive in a competitive landscape. By leveraging powerful data analytics and strategic insights from CareSet, organizations can enhance their outreach efforts, leading to improved patient outcomes and optimized lifecycle management of pharmaceutical products.
Navigate Regulatory Challenges and Stakeholder Relationships
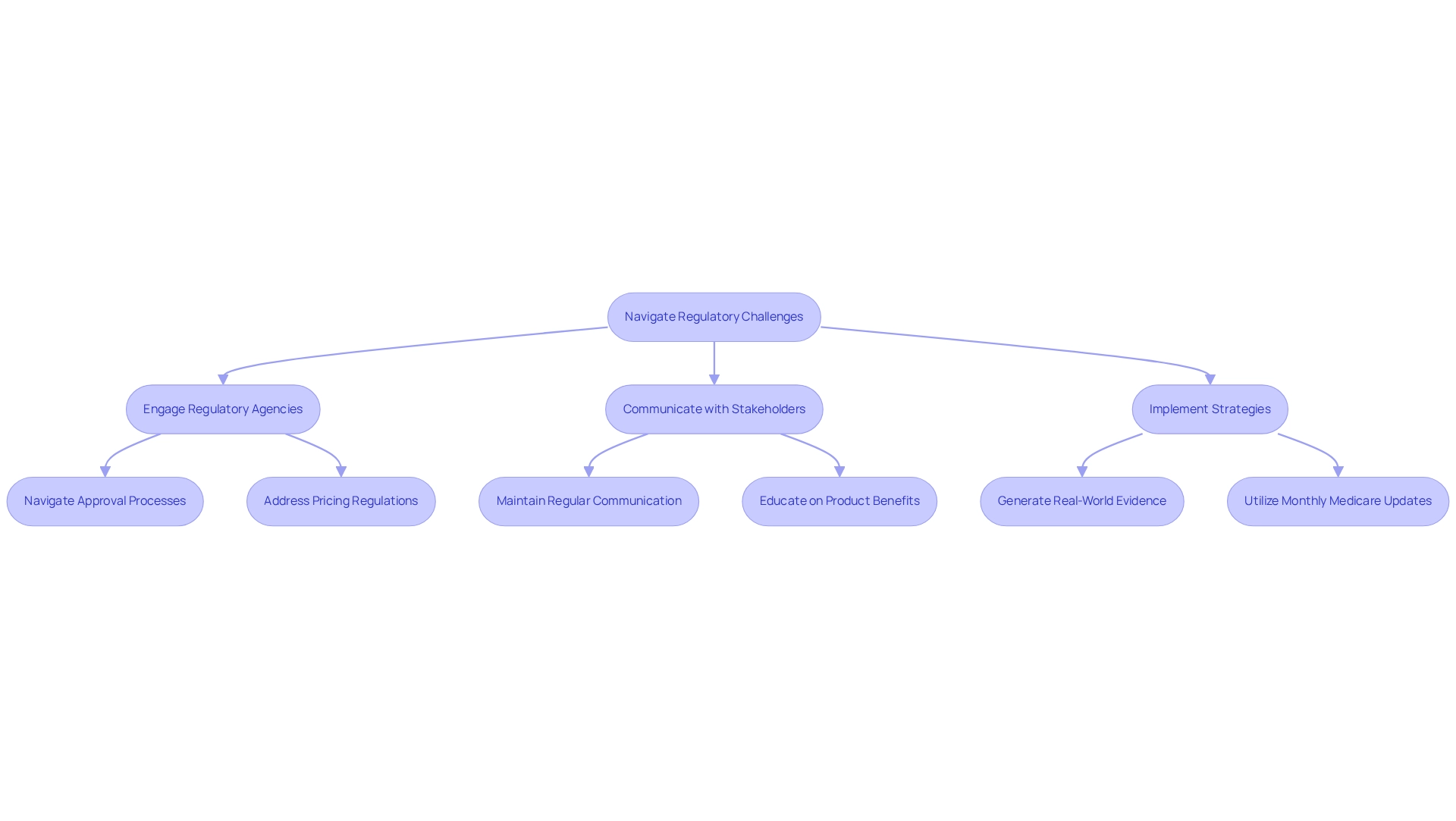
Pharmaceutical companies face a multitude of regulatory challenges that can significantly impede market entry. These challenges include intricate approval processes, stringent pricing regulations, and complex reimbursement policies. Delays in reimbursement approvals can hinder patient availability and exert financial pressure on pharmaceutical firms. Therefore, it is crucial to engage with regulatory agencies early in the product development process to navigate these challenges effectively. Establishing robust relationships with key stakeholders—such as payers, healthcare providers, and patient advocacy groups—plays a critical role in facilitating smoother negotiations and enhancing the understanding of a product’s value proposition.
Investing in effective stakeholder engagement approaches is paramount. This involves maintaining regular communication, educating stakeholders about the product’s benefits, and collaborating on the generation of real-world evidence to support reimbursement decisions. CareSet’s monthly Medicare updates provide valuable insights into drug utilization and treatment pathways, empowering pharmaceutical companies to enhance provider engagement and navigate the patient journey more effectively. For instance, early engagement with payers and policymakers can streamline approval processes and accelerate drug uptake, as demonstrated by companies that collaborated with key opinion leaders (KOLs) and advocacy groups from the development stage. Such proactive strategies not only alleviate regulatory challenges but also cultivate long-term collaborations that improve success in the marketplace.
Statistics indicate that 39% of social media users expect quick responses, underscoring the necessity for timely communication in stakeholder interactions. This expectation highlights the importance of being responsive and engaging effectively with stakeholders. Moreover, addressing patient adherence to treatment regimens through support programs can further improve outcomes related to market access in the pharmaceutical industry. Patients are more likely to follow prescribed treatments when they receive adequate support. As industry leaders emphasize, combining payer-reported utilization information with patient-level information is crucial for implementing value-based agreements. Michael Kerman, Vice President of Marketing, observes that “pharma organizations must create the ability to integrate combinations of payer-reported usage information with patient-level information from the channel, labs, and health systems to have any chance of implementing value-based agreements.” This underscores the significance of thorough information plans, like those provided by CareSet, in addressing industry entry obstacles.
Implement Strategies for Effective Market Access and Product Launch
To implement effective market access strategies, pharmaceutical companies must focus on several pivotal areas:
-
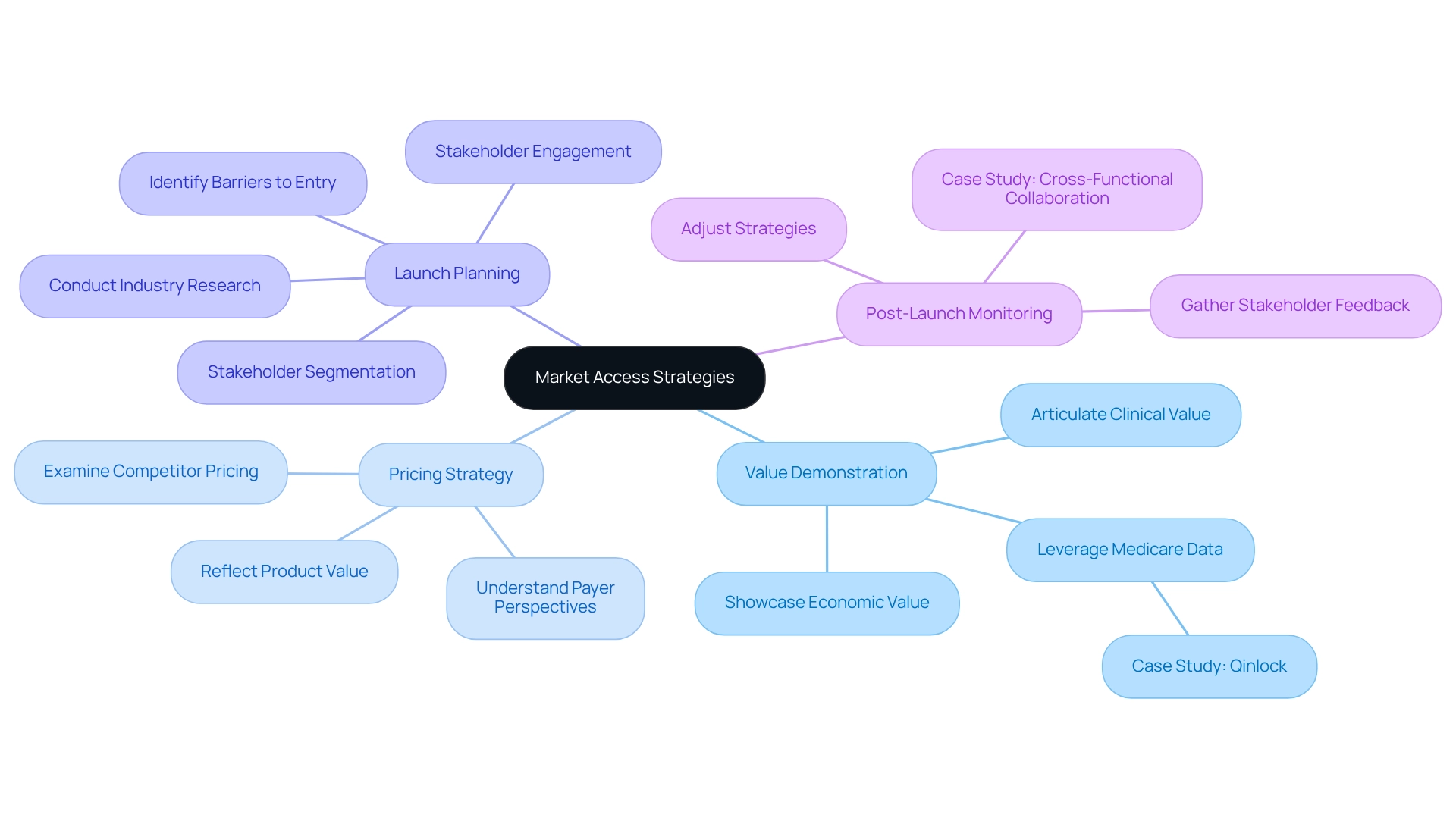
Value Demonstration: It is essential to clearly articulate the clinical and economic value of the product to stakeholders. This requires showcasing robust evidence on efficacy, safety, and cost-effectiveness, which is increasingly crucial as patients and advocacy groups play a central role in market access considerations. CareSet examines over $1.1 trillion in yearly Medicare claims data, underscoring the importance of evidence-based decision-making in demonstrating value. A case study titled “PUTTING PATIENTS FIRST: Unlocking Medicare Data to Empower HCP” on the oncology treatment Qinlock illustrates how leveraging Medicare data can enhance engagement with healthcare providers, particularly regarding late-stage treatment options for rare conditions like Gastrointestinal Stromal Tumor (GIST).
-
Pricing Strategy: Developing a pricing strategy that accurately reflects the product’s value while remaining competitive is critical. Understanding payer perspectives and their willingness to pay can significantly influence market access in the pharmaceutical industry. Streamlining this process involves examining industry trends and competitor pricing to ensure alignment with stakeholder expectations.
-
Launch Planning: A comprehensive launch strategy should encompass pre-launch activities, such as stakeholder engagement and thorough industry research. Identifying potential barriers to entry early on can facilitate a smoother introduction into the market. Stakeholder segmentation and mapping can provide insights for real-world evidence approaches post-launch, ensuring ongoing engagement with key players.
-
Post-Launch Monitoring: Continuous observation of marketplace performance following launch is essential. Gathering feedback from stakeholders allows for timely adjustments to strategies, effectively addressing emerging challenges. This iterative approach is supported by case studies, such as ‘Cross-Functional Collaboration for Launch Success,’ which highlight the importance of coordination among entry, brand, medical, and sales teams to ensure successful product launches.
By concentrating on these aspects, pharmaceutical firms can enhance their outreach strategies, ultimately leading to improved patient outcomes and refined product lifecycle management, which are crucial for effective market access in the pharmaceutical industry. As one expert noted, “Our research indicates that an archetype-based method to facilitate targeted promotional strategies may be more beneficial,” emphasizing the need for customized strategies in this complex environment.
Leverage Data Analytics for Enhanced Market Access Insights
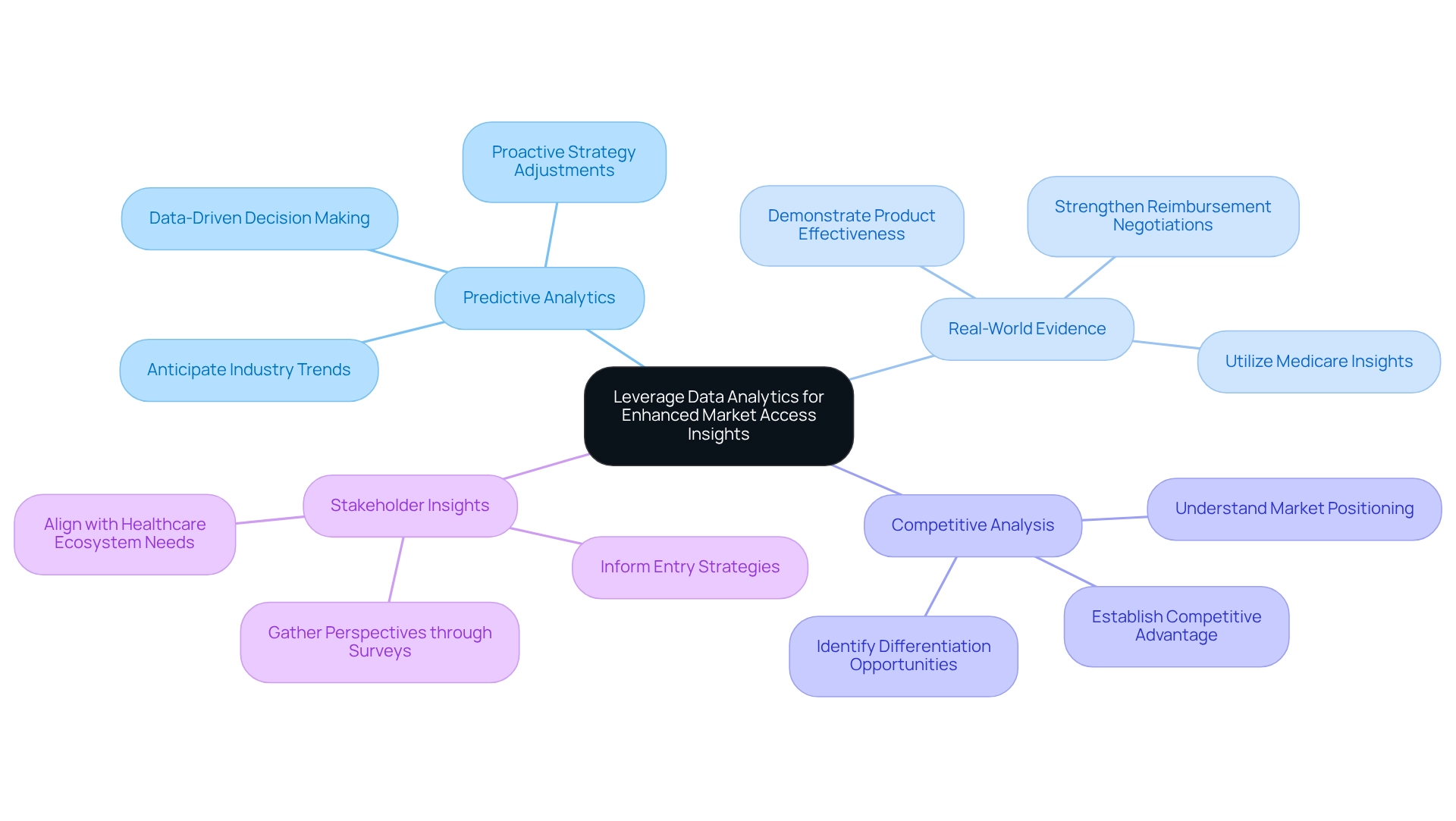
Utilizing analytics is vital for enhancing insights into the market access pharmaceutical industry. By harnessing various information sources, such as claims information, electronic health records, and research, companies can cultivate a nuanced understanding of treatment patterns, patient demographics, and payer behaviors. CareSet’s extensive Medicare data solutions, which encompass insights from over 62 million beneficiaries and 6 million providers, empower pharmaceutical and biotech firms to refine their strategies for market access in the pharmaceutical industry efficiently. Essential tactics include:
-
Predictive Analytics: Employ predictive modeling to anticipate industry trends and patient needs, facilitating proactive adjustments to entry strategies. This approach not only enhances responsiveness but also aligns with the increasing focus on data-driven decision-making within the industry.
-
Real-World Evidence: Gathering and analyzing real-world evidence is crucial for demonstrating a product’s effectiveness in everyday clinical settings. CareSet’s Medicare insights provide concrete proof of a product’s value in real-world applications, significantly strengthening reimbursement negotiations.
-
Competitive Analysis: Conducting thorough competitive evaluations helps organizations understand how similar products are positioned in the market. This insight enables the identification of differentiation opportunities, which are essential for establishing a competitive advantage.
-
Stakeholder Insights: Collecting insights from stakeholders through surveys and interviews is vital for grasping their perspectives. This information can inform entry strategies within the market access pharmaceutical industry, ensuring alignment with the needs and expectations of key participants in the healthcare ecosystem.
Incorporating analytics into entry planning empowers pharmaceutical companies to make informed decisions, ultimately increasing their chances of success. The impact of comprehensive analytics platforms, such as those provided by CareSet, is significant, with a typical return on investment realized within 14-18 months. Furthermore, the potential human and financial rewards of these analytics are substantial, promising considerable benefits for organizations that adopt them.
Case studies illustrate the effectiveness of these strategies; for instance, AstraZeneca’s integration of an analytics platform resulted in a 30% increase in candidate molecules progressing to clinical trials, showcasing the tangible benefits of data-driven approaches. As highlighted in Everest Group’s whitepaper, the integration of AI and Augmented Analytics is transforming the life sciences commercial function, making it imperative for companies to leverage these tools. Additionally, as Accenture noted, the importance of delivering relevant and personalized content cannot be overstated, particularly given the detrimental effects of irrelevant digital materials during the pandemic.
As the landscape of the market access pharmaceutical industry continues to evolve, the significance of predictive analytics and real-world evidence will only grow, shaping the future of healthcare data analytics.
Conclusion
The intricate landscape of market access in the pharmaceutical industry underscores its crucial role in ensuring that innovative therapies reach patients effectively and affordably. By grasping the multifaceted strategies involved, pharmaceutical companies can adeptly navigate the complexities of regulatory challenges and stakeholder relationships. Early engagement with payers and the leveraging of comprehensive data analytics are essential components that bolster market access efforts, ultimately leading to enhanced patient outcomes.
As the industry confronts the challenges posed by advanced biologics and monoclonal antibodies, the necessity of tailored market access strategies becomes increasingly apparent. Companies that proficiently demonstrate the clinical and economic value of their products, coupled with strategic pricing and launch planning, position themselves for success in a competitive marketplace. Continuous monitoring and adaptation post-launch are vital for addressing emerging barriers and ensuring sustained market presence.
The future of market access will be shaped by the integration of predictive analytics and real-world evidence, empowering pharmaceutical organizations to make informed decisions and optimize their strategies. By harnessing the power of data and nurturing strong stakeholder relationships, companies can not only enhance their market positioning but also fulfill their commitment to delivering innovative therapies to the patients who need them most. This holistic approach is essential for thriving in the evolving pharmaceutical landscape and ultimately improving health outcomes on a broader scale.


