Overview
Mastering inspection readiness is paramount for healthcare success, ensuring compliance with regulations, mitigating risks, and fostering a culture of continuous improvement. Effective practices such as:
- Thorough documentation
- Staff training
- Integration of technology
collectively enhance operational efficiency and significantly improve patient care outcomes. By implementing these strategies, healthcare organizations not only prepare for inspections but also position themselves for sustained excellence in service delivery.
Introduction
In the ever-evolving landscape of healthcare, the concept of inspection readiness has emerged as a critical component for organizations striving to navigate the complexities of regulatory compliance. This proactive strategy safeguards against potential penalties while enhancing operational efficiency and fostering a culture of continuous improvement. As healthcare regulations grow increasingly intricate and compliance costs rise, understanding the key elements of inspection readiness becomes essential for organizations aiming to maintain high standards of patient care and safety. By leveraging technological advancements and implementing best practices, the journey toward robust inspection readiness significantly impacts both organizational reputation and patient outcomes.
Understand the Importance of Inspection Readiness
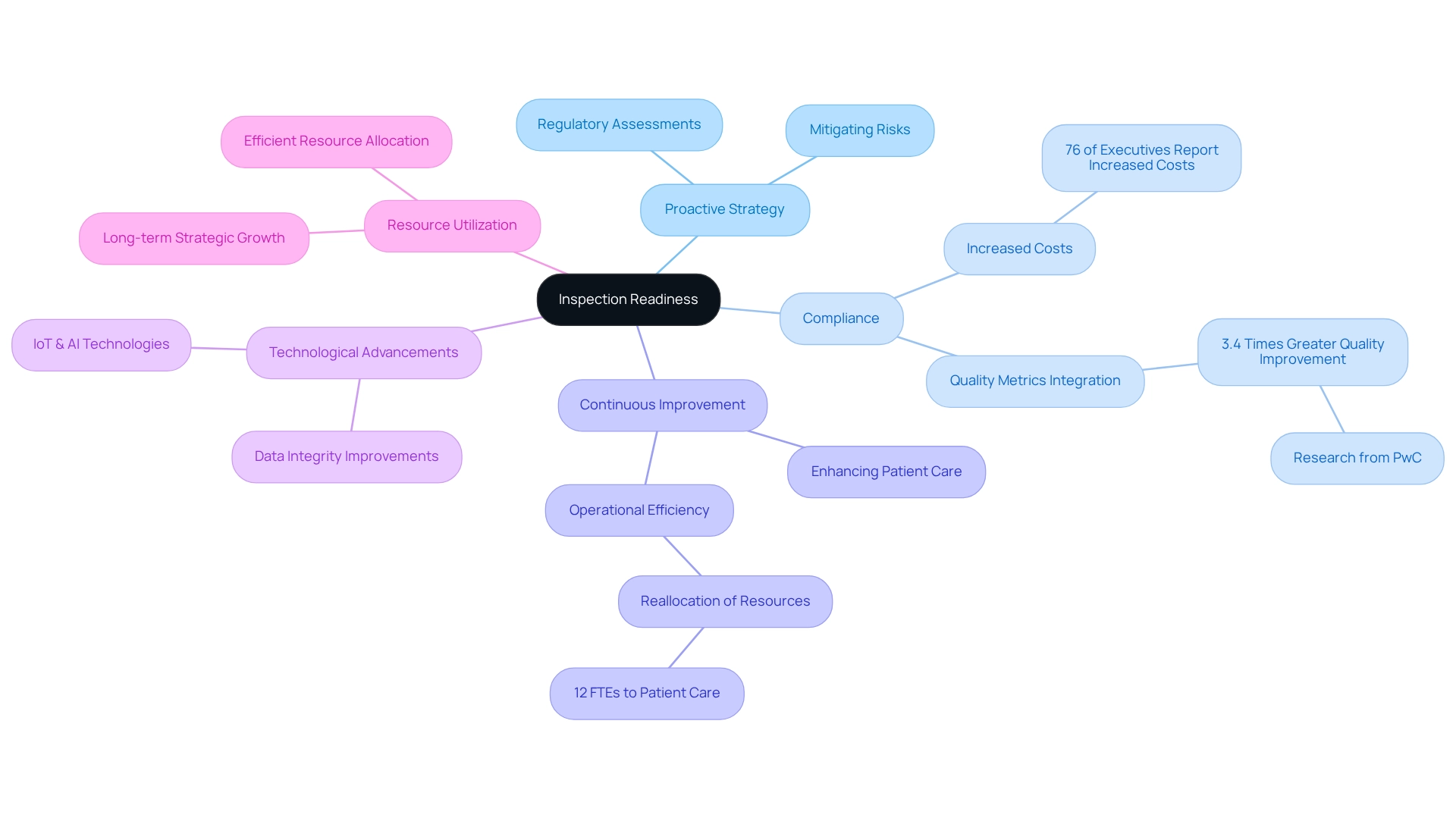
Preparation for evaluations represents a proactive strategy that empowers healthcare organizations to face regulatory assessments with confidence and effectiveness. This strategy encompasses a comprehensive range of practices designed to ensure compliance with healthcare regulations and standards. Organizations that maintain a state of compliance not only mitigate the risk of penalties and fines but also bolster their reputation among key stakeholders, including patients, providers, and regulatory authorities.
The advantages of being inspection-ready extend well beyond mere compliance; they cultivate a culture of continuous improvement within operational processes, ultimately enhancing inspection readiness, patient care, and safety. For example, a medium-sized hospital system successfully reallocated 12 full-time equivalent positions from manual reporting to patient care initiatives, illustrating how inspection readiness can lead to more efficient resource utilization.
Moreover, research indicates that pharmaceutical companies that integrate quality metrics into their daily operations achieve 3.4 times greater quality enhancement compared to those that regard metrics as sporadic activities. As noted by Sarah Lee, “Research from PwC found that pharmaceutical organizations that successfully embedded quality metrics into daily operations achieved 3.4 times greater quality improvement than those treating metrics as periodic reporting exercises.” This underscores the significant impact that preparedness for evaluations has on healthcare adherence rates and the overall quality of care.
In 2025, the importance of preparatory evaluations is underscored by the increasing complexity of healthcare regulations and the rising costs associated with compliance. A 2022 Deloitte survey revealed that 76% of healthcare leaders reported heightened regulatory expenses over the past three years, emphasizing the need for organizations to adopt effective assessment preparation strategies. The escalating compliance costs further highlight the necessity for proactive measures to avert potential penalties and enhance operational efficiency.
Technological advancements are also transforming inspection readiness for evaluations. Innovations such as IoT sensors, blockchain, and AI are enhancing data integrity and uncovering intricate patterns in quality metrics, enabling organizations to identify and address potential gaps ahead of evaluations. For instance, CareSet’s extensive healthcare data insights empower organizations to leverage these technologies effectively, ensuring they are well-prepared for evaluations. The case study titled ‘Technological Innovations in Quality Metrics’ demonstrates how these technologies are utilized to improve data collection and analysis, ultimately facilitating preparedness for evaluations. By prioritizing preparation for evaluations and harnessing CareSet’s data solutions, healthcare organizations can not only ensure compliance but also foster long-term strategic growth and improve patient outcomes.
Identify Key Components of Inspection Readiness
Key components of inspection readiness encompass several essential practices.
-
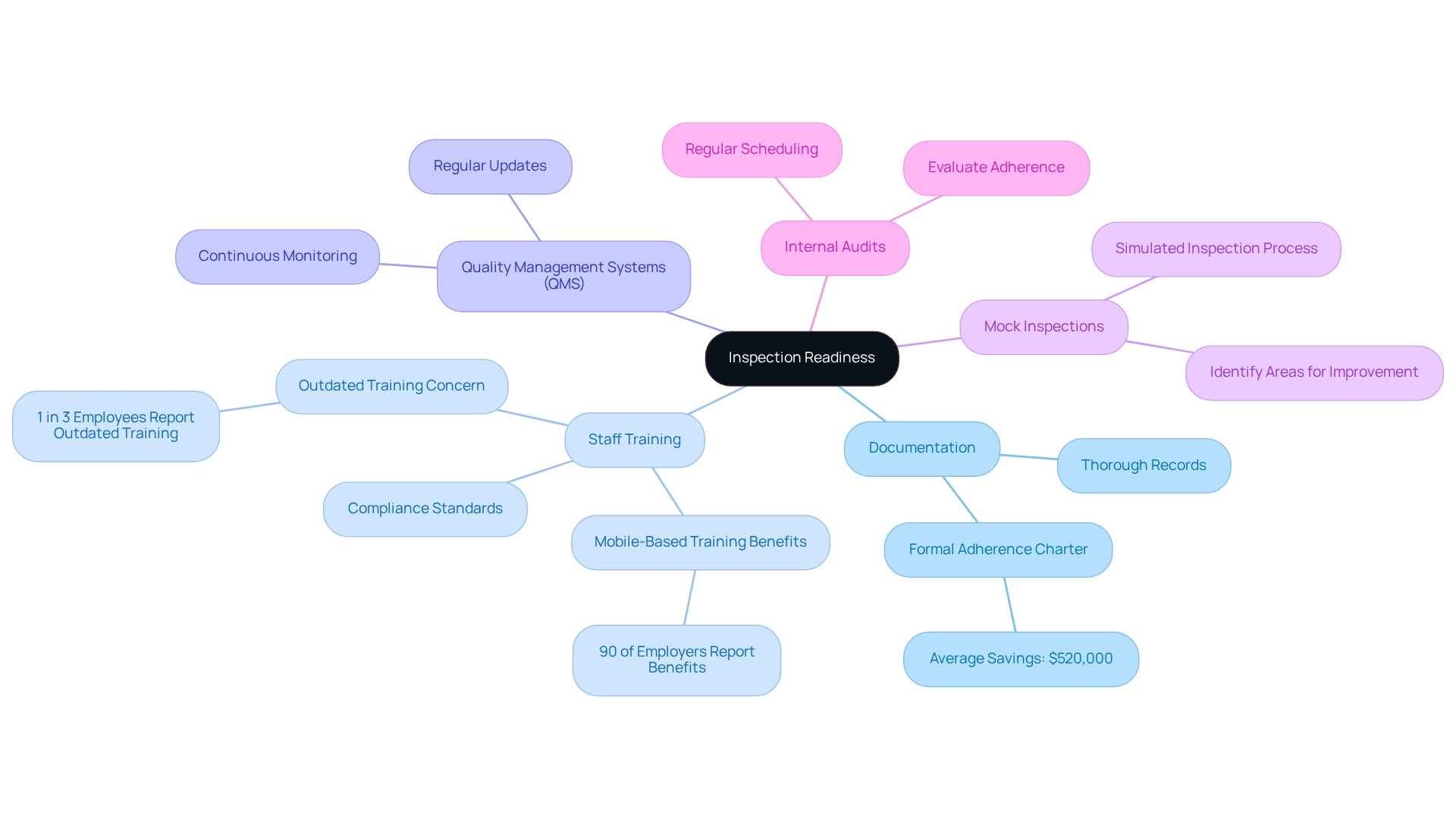
Documentation: Maintaining thorough and accurate records is crucial. This includes comprehensive documentation of processes, policies, procedures, training records, audits, and corrective actions taken. Effective documentation not only aids in meeting regulations but also enhances operational transparency. The establishment of a formal adherence charter can yield substantial savings, with companies conserving an average of $520,000.
-
Staff Training: It is vital that all staff members are well-versed in compliance standards. Frequent training sessions bolster understanding and clarify responsibilities during evaluations. Notably, 90% of employers report benefits from transitioning to mobile-based training, which can enhance engagement and retention. However, it is concerning that 1 in 3 employees state their organization’s training is outdated, underscoring the necessity for regular updates.
-
Quality Management Systems (QMS): A robust QMS should be implemented and regularly updated to align with current regulations and best practices. This system facilitates continuous monitoring and improvement, ensuring that processes remain compliant and effective.
-
Mock Inspections: Conducting regular mock inspections simulates the actual inspection process, helping to identify areas for improvement and preparing staff for real inspections. This proactive approach can significantly enhance readiness and confidence.
-
Internal Audits: Scheduling regular internal audits is essential for evaluating adherence to established protocols. These audits assist in recognizing shortcomings that require attention, ultimately resulting in enhanced adherence to regulations and operational efficiency.
Incorporating these elements not only fosters inspection readiness within organizations but also promotes a culture of adherence and ongoing enhancement. For example, companies that adopted regulatory monitoring saved an average of $1.03 million, highlighting the financial advantages of proactive adherence strategies. Moreover, as emphasized in recent findings, 50% of risk and adherence professionals characterized their programs as advanced, signifying an increasing acknowledgment of the significance of thorough regulatory frameworks. Additionally, two-thirds of corporate risk and compliance professionals agree on the duty to address ESG-related issues, reflecting the evolving landscape of compliance in healthcare.
Implement Best Practices for Preparation
To effectively prepare for evaluations, organizations must adopt the following best practices:
-
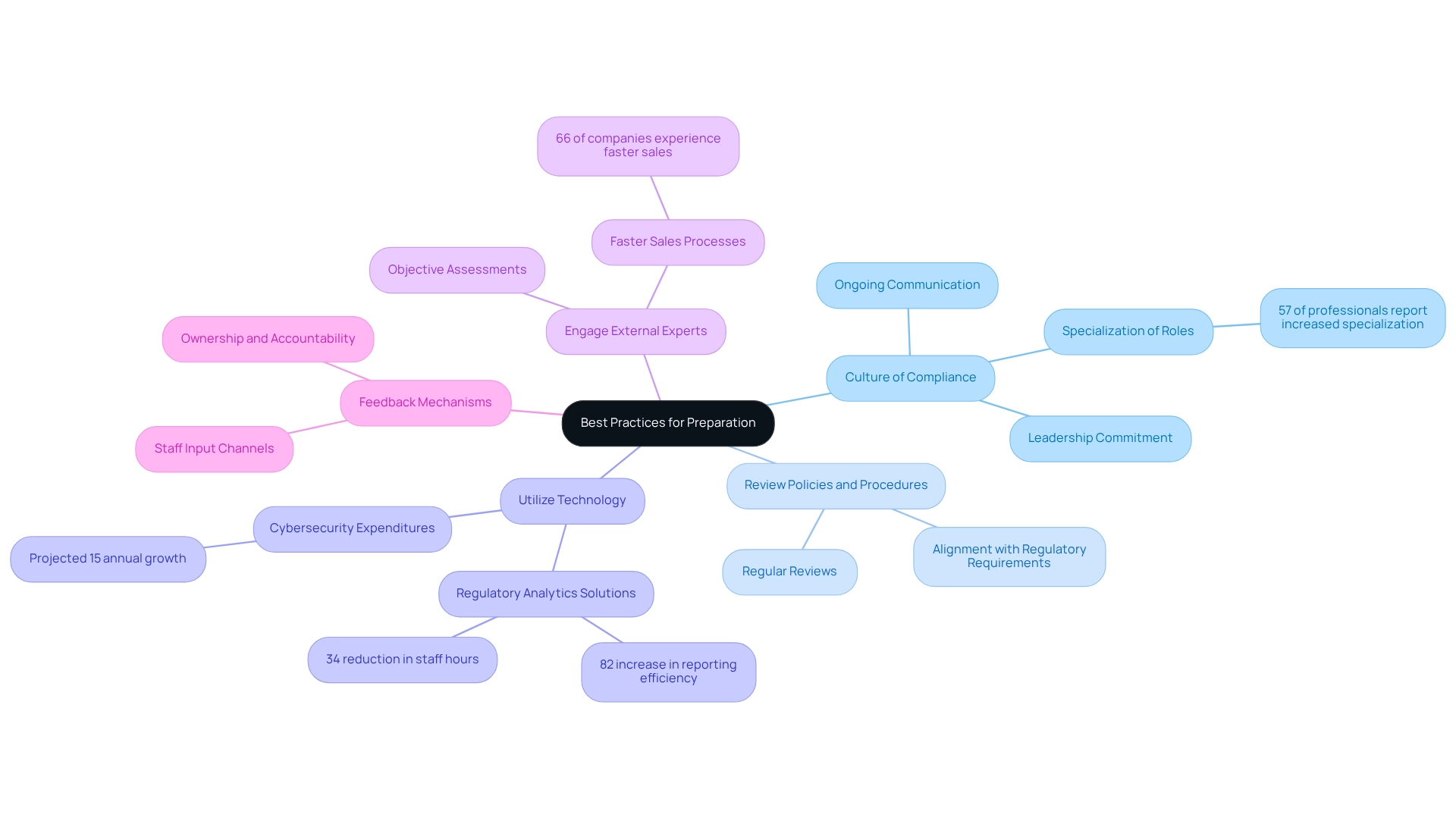
Establish a Culture of Compliance: Cultivating a compliance-centric environment is essential. Securing leadership commitment and fostering ongoing communication about the significance of ensuring inspection readiness is crucial. A robust culture of adherence not only enhances conformity to regulations but also empowers employees to prioritize quality and accountability in their roles. Notably, 57% of corporate risk and regulatory professionals indicate that such roles have become more specialized, underscoring the necessity for organizations to adapt their strategies accordingly.
-
Regularly Review Policies and Procedures: Keeping policies and procedures current is vital for compliance. Organizations should conduct regular reviews to ensure alignment with evolving regulatory requirements. This proactive approach minimizes the risk of non-compliance and prepares teams for inspections by ensuring familiarity with the latest standards.
-
Utilize Technology: Embracing technology can significantly enhance documentation and reporting processes. Implementing electronic systems improves the accuracy and accessibility of records, streamlining workflows and reducing the likelihood of errors. For instance, healthcare organizations that adopt regulatory analytics solutions have reported an 82% increase in reporting efficiency while reducing staff hours by 34%, allowing for a greater focus on patient care initiatives. Furthermore, with healthcare cybersecurity expenditures projected to increase by approximately 15% each year, investing in technology is essential for upholding regulations and protecting sensitive information.
-
Engage External Experts: Bringing in external consultants or experts can provide valuable insights into best practices for ensuring inspection readiness. Their objective assessments can identify potential blind spots that internal teams might overlook, ensuring a more thorough preparation process. This can result in quicker sales cycles, as evidenced by UserEvidence’s finding that 66% of companies experience faster sales processes when adherence is prioritized.
-
Feedback Mechanisms: Establishing channels for staff input on adherence processes fosters a sense of ownership and accountability. Motivating staff to express their perspectives can uncover opportunities for enhancement and improve overall readiness for evaluations.
By adopting these optimal methods, healthcare entities can not only prepare efficiently for reviews but also cultivate a lasting culture of adherence that promotes long-term operational success. Moreover, organizations such as IMDRF and MDSAP are striving for standardized regulations across various markets, which can further assist in aligning compliance efforts with global standards.
Troubleshoot Common Inspection Readiness Challenges
Common challenges in achieving inspection readiness include:
-
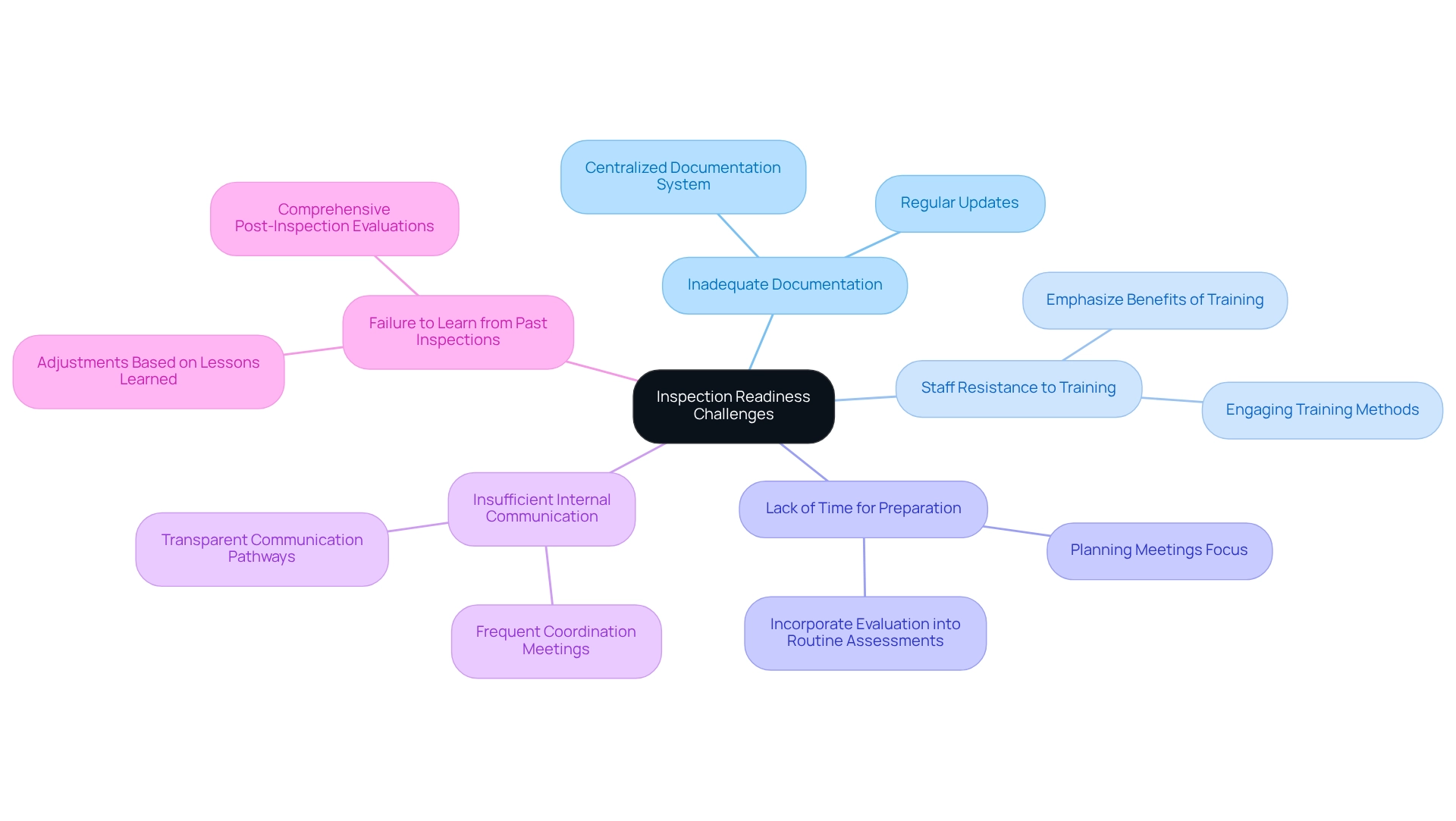
Inadequate Documentation: Many organizations struggle to maintain comprehensive documentation. Implementing a centralized documentation system that is regularly updated and easily accessible can significantly improve this aspect. For instance, Memorial Health System’s adoption of an integrated regulatory analytics platform resulted in a 78% decrease in false-positive alerts, demonstrating the effectiveness of strategic solutions in addressing documentation challenges.
-
Staff Resistance to Training: Employees may oppose training initiatives, which can obstruct adherence efforts. Emphasizing the benefits of training for personal development and organizational success, along with incorporating engaging training methods, can help overcome this resistance. As the compliance landscape evolves, organizations must adapt their training approaches to meet new standards.
-
Lack of Time for Preparation: Organizations often find it challenging to allocate sufficient time for readiness amidst daily operations. To emphasize evaluation preparedness, incorporate it into routine operational assessments and planning meetings, ensuring it stays a central focus.
-
Insufficient Internal Communication: Poor communication can lead to misunderstandings regarding evaluation processes. Creating transparent communication pathways and conducting frequent meetings can ensure all personnel are updated and coordinated on evaluation preparation initiatives.
-
Failure to Learn from Past Inspections: Organizations may repeat mistakes from previous inspections. Carrying out comprehensive post-inspection evaluations to recognize lessons learned and making adjustments can avert recurrence and improve future preparedness.
Statistics show that 49% of survey participants anticipate heightened personal liability for regulatory professionals, highlighting the significance of tackling these challenges in advance. Moreover, the reduction in entities attaining complete PCI compliance—from 43.4% in 2020 to 14.3% in 2023—emphasizes the essential requirement for upholding compliance and preparedness. By addressing these common challenges, organizations can improve their inspection readiness and ultimately enhance patient outcomes.
Conclusion
In the dynamic realm of healthcare, the significance of inspection readiness cannot be overstated. This proactive approach not only prepares organizations for regulatory scrutiny but also enhances their operational efficiency and fosters a culture of continuous improvement. By implementing key components such as:
- Thorough documentation
- Regular staff training
- Robust quality management systems
healthcare organizations can navigate the complexities of compliance with greater confidence. The integration of technological innovations further streamlines processes, enabling organizations to identify and rectify potential issues before they escalate into compliance failures.
The journey toward inspection readiness is marked by the adoption of best practices that cultivate a culture of compliance. Establishing a commitment from leadership, utilizing technology to enhance documentation, and engaging external experts can significantly bolster an organization’s readiness for inspections. Moreover, addressing common challenges like inadequate documentation and staff resistance to training is crucial for maintaining a high standard of compliance. As the healthcare landscape continues to evolve, organizations that prioritize inspection readiness will not only safeguard their reputation but also ensure the highest levels of patient care and safety.
Ultimately, the focus on inspection readiness serves as a foundational strategy for healthcare organizations aiming to thrive in an increasingly regulated environment. By embracing a proactive mindset and leveraging best practices, organizations can enhance their operational success, improve patient outcomes, and position themselves as leaders in the healthcare industry. The time for action is now; investing in inspection readiness is an investment in the future of healthcare.

