Overview
Key insights from pan tumor analysis reveal that the integration of data across various cancer types can significantly enhance patient outcomes by tailoring treatment strategies to individual needs. This analysis captures attention by underscoring the critical role of comprehensive data in modern oncology.
The article builds interest by illustrating how understanding demographic variations, immune subtypes, and biomarkers leads to more effective and personalized therapies. Such insights generate a desire for improved care protocols, ultimately aiming to enhance survival rates across diverse malignancies.
By embracing these strategies, healthcare providers can take actionable steps towards delivering superior patient care.
Introduction
In the realm of oncology, the quest for personalized treatment strategies has never been more crucial. As researchers delve deeper into the complexities of cancer, innovative methodologies like pan-tumor analysis are shedding light on treatment patterns across various cancer types. These revelations provide vital insights that can transform patient care. By examining the interplay of immune subtypes, multi-omic data, and biomarkers, healthcare professionals are better equipped to tailor therapies that align with individual patient profiles.
This article explores the latest advancements in understanding treatment pathways, the impact of immune responses on outcomes, and the pivotal role of biomarkers in developing targeted therapies. As the landscape of cancer treatment evolves, these insights promise to enhance therapeutic efficacy and improve survival rates for patients facing this formidable disease.
Understand Treatment Patterns through Pan-Tumor Analysis
The analysis of pan tumor data integrates information from various cancer types, yielding essential insights into care patterns. This methodology reveals common therapies, response rates, and discrepancies in care protocols, thereby enabling healthcare professionals to tailor care plans effectively.
For example, recent studies suggest that specific therapies can result in significantly improved outcomes for particular demographics, highlighting the necessity of personalized approaches. Notably, nearly half (49%) of Hodgkin Lymphoma (HL) cases occur in individuals under 40 years, while 87% of Non-Hodgkin Lymphoma (NHL) cases are identified in adults aged 50 years or older, underscoring the need for age-specific management strategies.
Furthermore, approximately 10% of individuals who have survived illness continue to smoke even up to 9 years post-diagnosis, which can adversely affect recovery outcomes. By leveraging extensive Medicare data, stakeholders can pinpoint successful treatment strategies and refine care pathways. This not only enhances health outcomes but also fosters a more efficient healthcare system, ultimately benefiting individuals across diverse malignancies.
Moreover, the ongoing NILE trial, which assesses durvalumab with or without tremelimumab alongside platinum-based chemotherapy, exemplifies the innovative treatments under investigation to improve outcomes for individuals with advanced urothelial carcinoma. Insights derived from CareSet’s comprehensive Medicare data, including the case study on Qinlock for Gastrointestinal Stromal Tumor (GIST), illustrate how understanding care pathways can empower healthcare providers to engage more effectively with individuals and broaden therapeutic options.
The insights gained from pan tumor analysis are pivotal for developing effective and well-tolerated therapies, thus addressing the high attrition rates in oncology.
Explore the Impact of Immune Subtypes on Patient Outcomes
Studies indicate that biological variations significantly influence individual outcomes in oncological care. Patients classified as having an ‘immune-hot’ subtype generally show higher response rates to immunotherapies than their ‘immune-cold’ counterparts. This distinction is vital, as it enables healthcare providers to predict treatment responses with greater accuracy and tailor therapies to meet individual patient needs.
For example, a recent study titled ‘Impact of Pan Tumor Cellular Microenvironment on Cancer Progression’ investigated how pan tumors interact with the cellular microenvironment and its effect on cancer advancement in gynecologic cancers. The findings underscored the critical role of cell composition in either promoting or inhibiting pan tumor growth, suggesting that a deeper understanding of these interactions could guide future therapeutic strategies.
Moreover, real-world examples illustrate how customized treatment strategies, derived from biological profiling, can enhance therapeutic effectiveness while minimizing adverse effects. In 2025, statistics revealed that individuals with immune-hot profiles experienced significantly improved outcomes, with a notable difference calculation for amplification related to immunomodulator deletions. This highlights the necessity of incorporating subtype analysis into clinical practice.
As Farshad Farshidfar stated, ‘To ascertain whether the observed associations are derived by specific molecular subtypes, we repeated this analysis using the molecular subtypes previously identified by the TCGA tumor-specific studies instead of tumor tissue of origin.’
As research advances to uncover the complexities of immune responses, the ability to differentiate between immune subtypes will be essential for optimizing patient management and enhancing care.
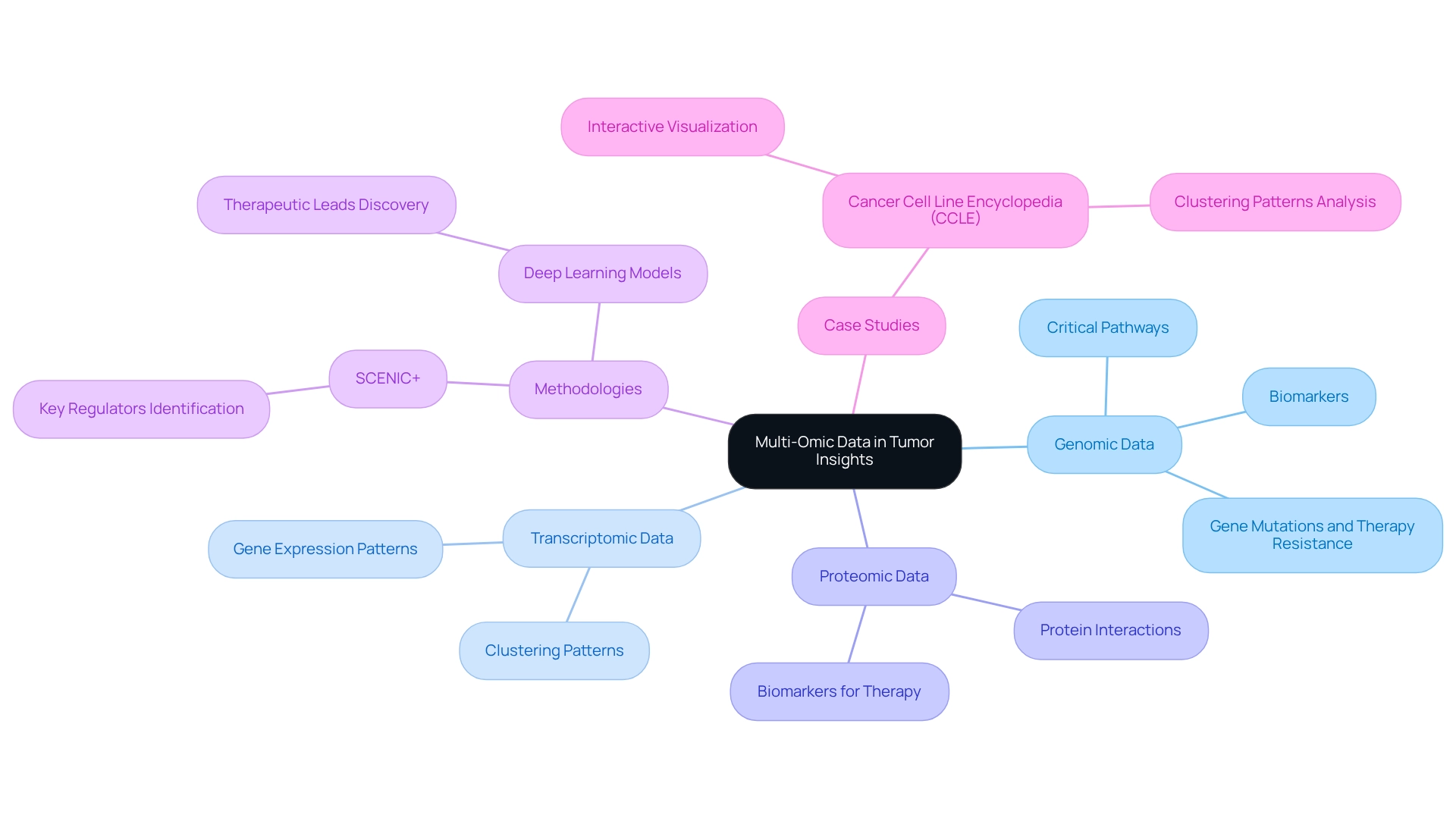
Leverage Multi-Omic Data for Comprehensive Tumor Insights
Leveraging multi-omic data provides researchers with a comprehensive perspective on tumor biology. By synthesizing genomic, transcriptomic, and proteomic information, scientists can identify critical molecular pathways and biomarkers that influence tumor progression. Recent studies reveal a strong correlation between specific gene mutations and resistance to therapy, highlighting the necessity for targeted approaches.
As Vasilis Konstantakos and Seppe De Winter articulated, “Through the comparison of gene regulatory programs across diseases using SCENIC+ and deep learning models, we aim to find key regulators per cell state and pave the way for discovering more specific biomarkers and new leads for therapeutic intervention.”
This integrative approach not only enhances the understanding of pan tumor dynamics but also facilitates the development of personalized treatment strategies, ultimately leading to improved patient outcomes.
For example, the Cell Line Encyclopedia (CCLE) project utilized interactive visualization tools to analyze gene expression data, uncovering clustering patterns among tumor cell lines that could inform therapeutic interventions. The CCLE Explorer enabled the identification of these clustering patterns, revealing potential mislabeling and unique mechanisms within specific sub-histologies, thereby deepening the understanding of gene expression in research.
Such insights are crucial for advancing cancer research and enhancing the effectiveness of intervention protocols. This work was partially supported by the NIH Common Fund.
Validate Immune Subtypes and Their Clinical Associations
Clinical validation of biological subtypes is essential for linking these classifications with individual outcomes and treatment responses. Studies demonstrate that individuals exhibiting specific biological profiles show markedly varied survival rates and responses to immunotherapy. For instance, in the IMvigor210 group, individuals with elevated scores displayed significantly better survival rates compared to those with low scores. This underscores the necessity for further research into checkpoint blockade therapies, particularly in pan tumor patients, to fully comprehend the impact of biological profiles on therapeutic outcomes.
A notable case study on colorectal malignancy (CRC) involved functional enrichment analysis of differentially expressed genes (DEGs) across various subtypes. This analysis unveiled critical pathways associated with immune system modulation, such as:
- Cytokine-cytokine receptor interactions
- Adaptive responses
These insights can inform treatment strategies by targeting these pathways. The validation set included transcriptome profiles and clinical data from 177 CRC samples, highlighting the robustness of these findings and their relevance to clinical practice.
Moreover, professional perspectives suggest that the biological profile may serve as a pivotal factor in outcomes across different tumor types. Bailiang Li from the Department of Radiation Oncology at Stanford University School of Medicine stated, “The profile of the body’s defenses may be a key determinant of outcomes across tumor types and could potentially be integrated into future biomarker-based risk stratification strategies for personalized therapy of HPV-related malignancies.” Integrating immune profiling into standard clinical practice is vital for enhancing therapeutic strategies and improving survival rates among cancer patients.
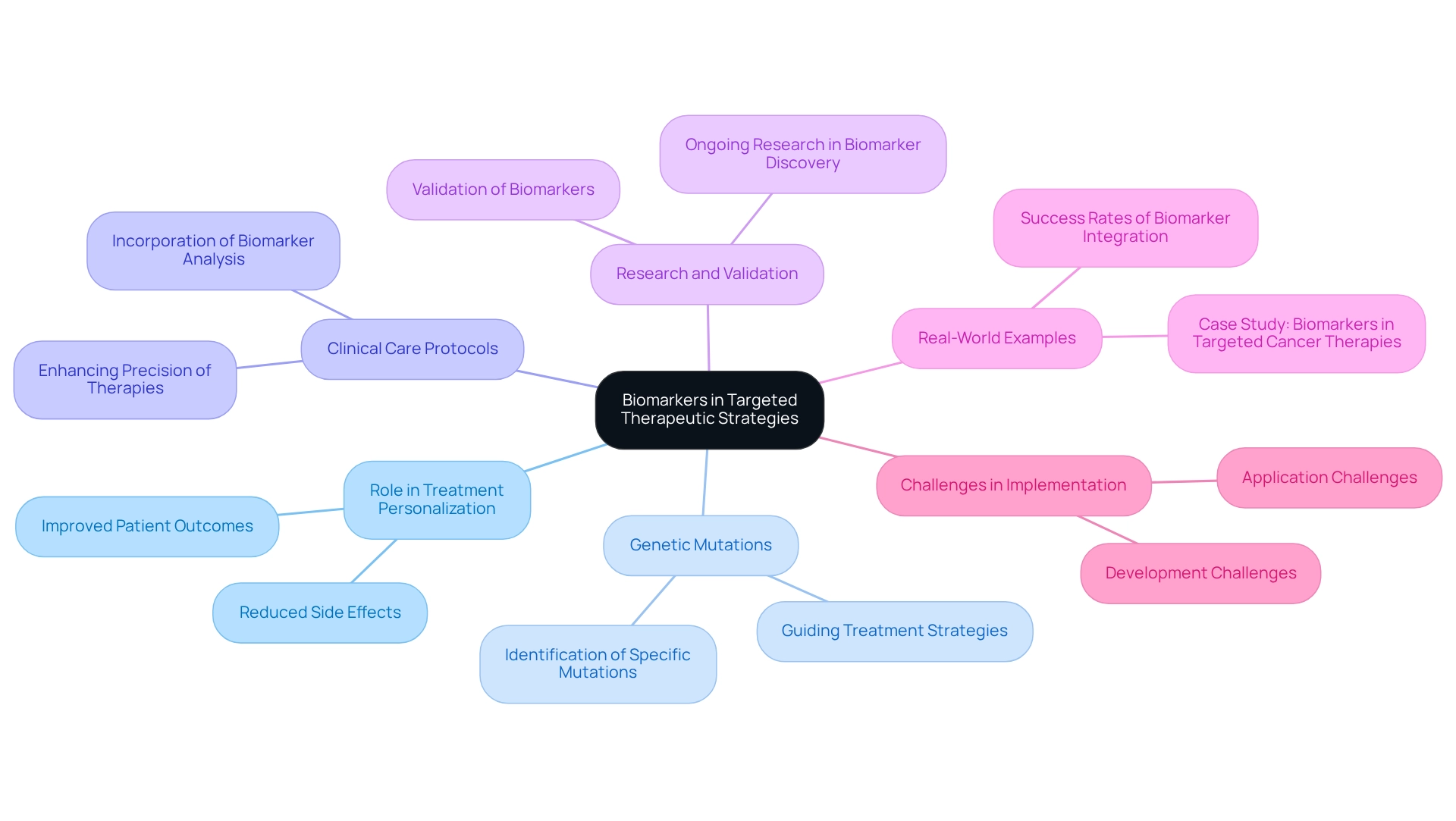
Identify Biomarkers for Targeted Therapeutic Strategies
Biomarkers play a pivotal role in the advancement of targeted therapies, facilitating the personalization of treatment options to align with individual patient profiles. The identification of specific genetic mutations is essential, as it informs the selection of targeted therapies that are more likely to be effective for particular patients. By incorporating biomarker analysis into clinical care protocols, healthcare professionals can significantly enhance the precision of therapies for malignancies, resulting in improved patient outcomes and reduced side effects. This methodology underscores the importance of ongoing research in biomarker discovery and validation, while also highlighting the transformative progress in pan tumor treatment.
Recent studies reveal that targeted therapies have fundamentally changed patient care, especially for those who are unable to endure standard chemotherapy, despite the persistent challenges in their development and implementation. The common side effects associated with chronic myeloid leukemia (CML) therapy can adversely affect quality of life, emphasizing the necessity for precision in cancer therapies to mitigate these impacts. Furthermore, the integration of biomarkers into clinical practice has shown promising success rates, with real-world examples demonstrating how genetic mutations can effectively guide treatment strategies. For instance, the case study titled ‘Biomarkers in Targeted Cancer Therapies’ illustrates how the identification and application of biomarkers enhance the selection of appropriate therapies, contributing to improved management and outcomes in oncology.
As the field of oncology care continues to progress, the significance of biomarkers remains critical in optimizing therapeutic strategies and enhancing patient management. Significant advancements have been made in the identification and targeting of druggable mutations, reinforcing the role of biomarkers in improving therapies for pan tumors. Ultimately, it is essential to acknowledge the ongoing challenges in the development and application of targeted therapies, as they will shape the future landscape of cancer treatment.
Conclusion
The exploration of personalized treatment strategies in oncology underscores the critical importance of understanding treatment patterns through pan-tumor analysis. This innovative approach aggregates data across various cancer types, revealing essential insights into effective therapies and demographic-specific treatment protocols. By leveraging such data, healthcare professionals can better tailor treatment plans, ultimately enhancing patient outcomes and fostering a more efficient healthcare system.
Moreover, the impact of immune subtypes on patient outcomes cannot be overstated. Differentiating between ‘immune-hot’ and ‘immune-cold’ profiles allows for more accurate predictions of treatment responses, enabling customized therapeutic approaches. As research continues to unveil the complexities of immune interactions within the tumor microenvironment, integrating immune profiling into clinical practice emerges as a pivotal strategy for optimizing cancer treatment.
The utilization of multi-omic data enriches our understanding of tumor biology, facilitating the identification of key molecular pathways and biomarkers. This comprehensive analysis not only aids in discovering targeted therapies but also enhances the efficacy of treatment protocols. As advancements in biomarker identification continue to transform cancer treatment, the focus on precision medicine becomes increasingly vital for improving patient management and survival rates.
In summary, the evolving landscape of cancer treatment emphasizes the necessity of personalized approaches grounded in robust data analysis. By harnessing insights from pan-tumor analysis, immune profiling, and multi-omic data, healthcare providers can significantly improve therapeutic efficacy, ultimately leading to better outcomes for patients facing the challenges of cancer. The commitment to ongoing research and innovation in these areas is essential for shaping the future of oncology and enhancing the lives of those affected by this formidable disease.


