Overview
The article delineates the pivotal trends in pharmaceuticals that are shaping market access strategies, underscoring the critical role of data-driven insights, regulatory changes, and innovative pricing models. It emphasizes the necessity for drug manufacturers to leverage Medicare data, adopt value-based pricing, and adeptly navigate regulatory landscapes. These elements are essential for enhancing market positioning and improving patient access to medications. By understanding these dynamics, stakeholders can better strategize their approaches in an evolving market.
Introduction
In the rapidly evolving landscape of the pharmaceutical industry, innovative strategies are fundamentally reshaping how companies approach market access and patient care. By harnessing the power of Medicare data insights, organizations are driving informed decision-making. Moreover, the embrace of value-based pricing aligns drug costs with patient outcomes, marking a transformative shift within the sector.
As artificial intelligence accelerates drug development and personalized medicine tailors treatments to individual needs, pharmaceutical firms are navigating new regulatory challenges while prioritizing sustainability and mental health solutions. With mergers and acquisitions on the rise, understanding these dynamics is crucial for stakeholders aiming to thrive in an increasingly competitive environment.
This article delves into the multifaceted strategies defining the future of pharmaceuticals, offering insights on how companies can adapt and succeed in this complex ecosystem.
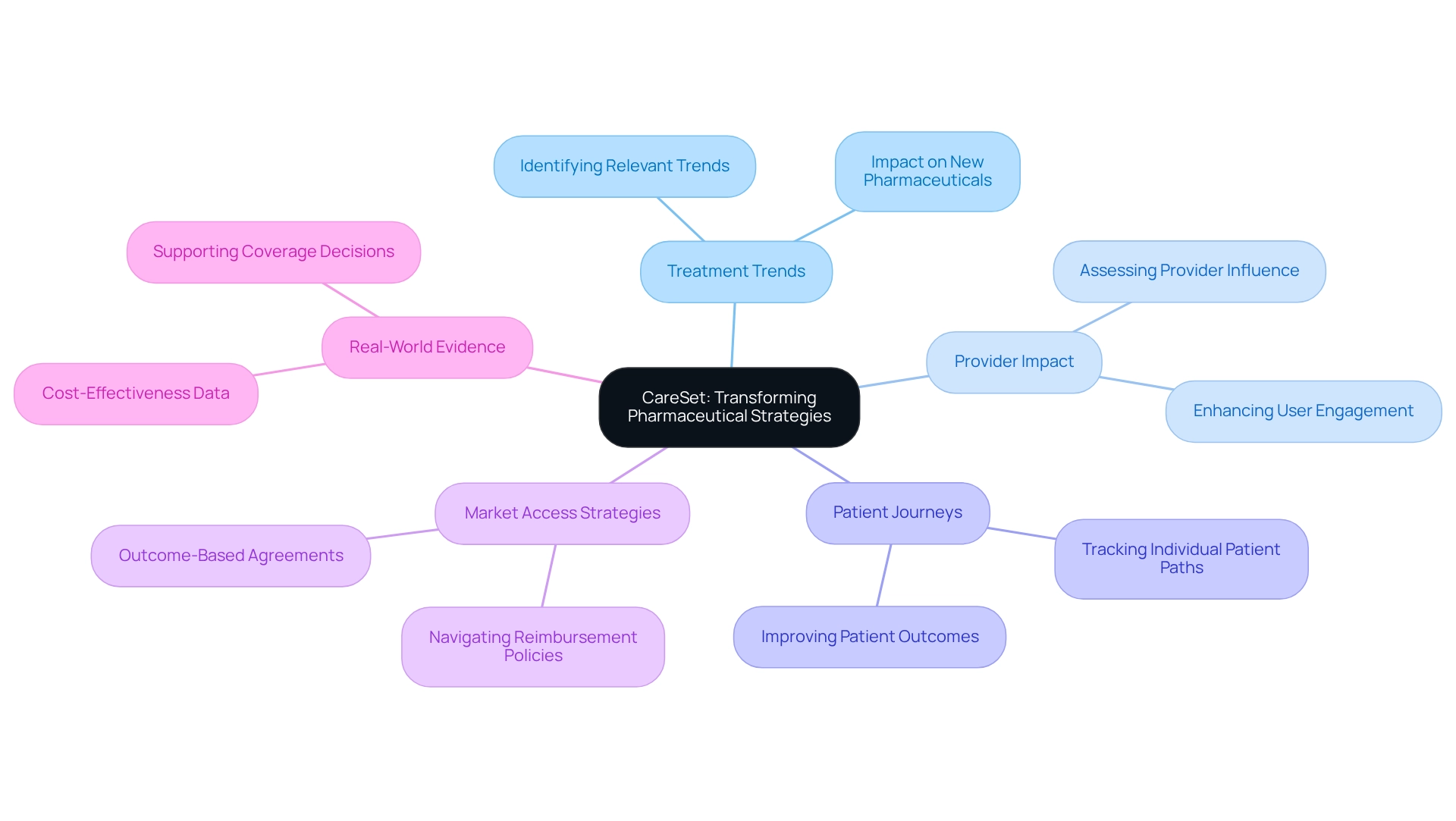
CareSet: Transforming Pharmaceutical Strategies with Medicare Data Insights
CareSet has fundamentally transformed the healthcare landscape by leveraging extensive Medicare claims information to deliver actionable insights. By evaluating over $1.1 trillion in annual claims and incorporating more than 100 external information sources, CareSet empowers drug manufacturers to:
- Identify treatment trends relevant to new pharmaceuticals
- Assess provider impact
- Track individual patient journeys
This information-centric approach enables stakeholders to make informed decisions and enhances market access strategies, ultimately improving outcomes for patients.
The integration of Medicare information into strategic planning is crucial for navigating the complexities of the healthcare ecosystem. It enables drug manufacturers to effectively direct their efforts, enhance user engagement, and address the challenges posed by intricate reimbursement policies. As highlighted in the case study ‘PUTTING PATIENTS FIRST: Unlocking Medicare Data to Empower HCP,’ even after receiving FDA or EMA approval, securing reimbursement for a drug can be arduous due to unpredictable reimbursement policies. Payers require real-world evidence and cost-effectiveness data before granting coverage, underscoring the need for robust market access strategies in new pharmaceuticals.
As the industry shifts towards outcome-based agreements, the significance of detailed Medicare insights escalates in new pharmaceuticals, ensuring that companies can secure reimbursement and facilitate access to essential medications. In 2025, the impact of Medicare claims data on drug market access strategies is more pronounced than ever, with organizations increasingly depending on real-world evidence and cost-effectiveness information from new pharmaceuticals to shape their approaches.
Jayne Hornung, Chief Clinical Officer, observes, ‘The shift from traditional volume-based contracts to outcome-based agreements will certainly continue.’ CareSet’s dedication to providing high-quality, comprehensive insights positions its clients to excel in this evolving landscape, fostering long-term strategic growth and enhancing the overall effectiveness of their market access initiatives.
Value-Based Pricing: Aligning Drug Costs with Patient Outcomes
Value-Based Pricing: Aligning Drug Costs with Patient Outcomes
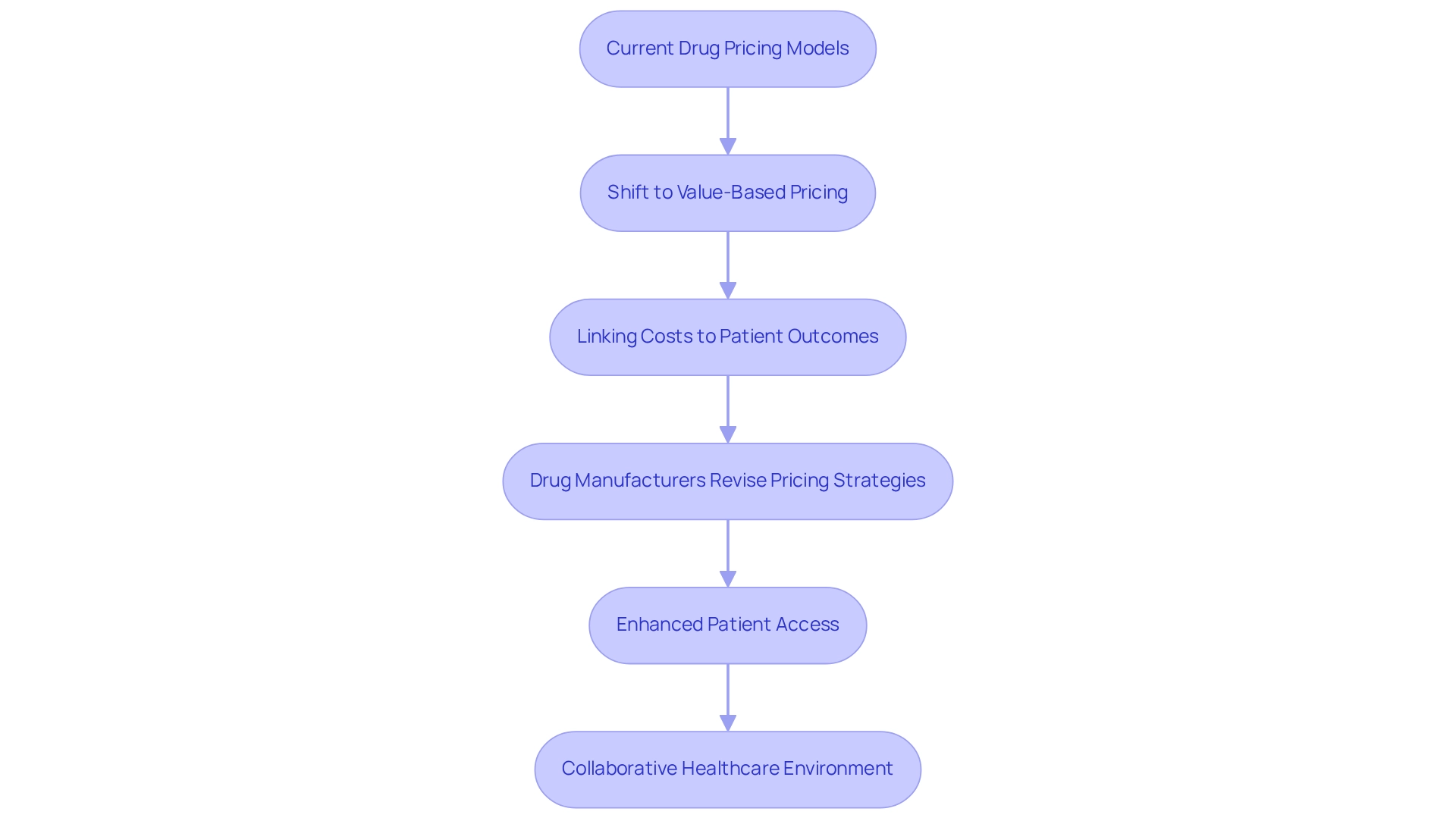
Value-based pricing is fundamentally transforming the pharmaceutical landscape by directly linking drug costs to patient outcomes. This innovative model compels manufacturers to substantiate the effectiveness of their products, ensuring individuals receive treatments that deliver genuine value. As healthcare systems increasingly adopt value-based care, drug manufacturers are compelled to revise their pricing strategies, reflecting the trends in news pharmaceuticals to meet these evolving expectations.
By 2025, statistics suggest that approximately 60% of drug manufacturers will adopt value-based pricing models, with many reporting enhanced patient access to essential medications. For instance, organizations implementing these models have experienced improved market positioning and heightened trust among stakeholders in the healthcare ecosystem. CareSet’s comprehensive healthcare information insights play a crucial role in this transformation, empowering pharmaceutical and biotech companies to leverage actionable insights for informed decision-making throughout the product lifecycle.
A significant case study titled “Value-Based Pricing Approach” emphasizes the capacity of this pricing model to transform drug pricing by concentrating on the real advantages to individuals rather than merely potential profits. This shift not only promotes equitable access to medications but also aligns drug development more closely with public health needs, supported by CareSet’s Medicare insights that provide critical data for stakeholders.
Expert insights indicate that the effectiveness of value-based pricing models is evident in enhanced outcomes, as these strategies encourage news pharmaceuticals companies to emphasize the tangible benefits of their products. Todd Snelgrove notes, “The further development of a metric able to capture all tangible and intangible benefits of alternative offers in sourcing decisions will thus, in the end, build on the value quantification and pricing literature.” As the industry continues to evolve, the trend toward connecting drug costs to consumer outcomes is expected to gain momentum, fostering a more collaborative and transparent healthcare environment.
To explore how CareSet’s Medicare insights can enhance your value-based pricing strategies and improve access to medications, we invite you to initiate a discussion. Ongoing conversations about pricing strategies on ResearchGate encourage individuals to stay updated with the latest research in pricing and other scientific topics, further emphasizing the importance of adapting to these changes.
Artificial Intelligence: Accelerating Drug Development Processes
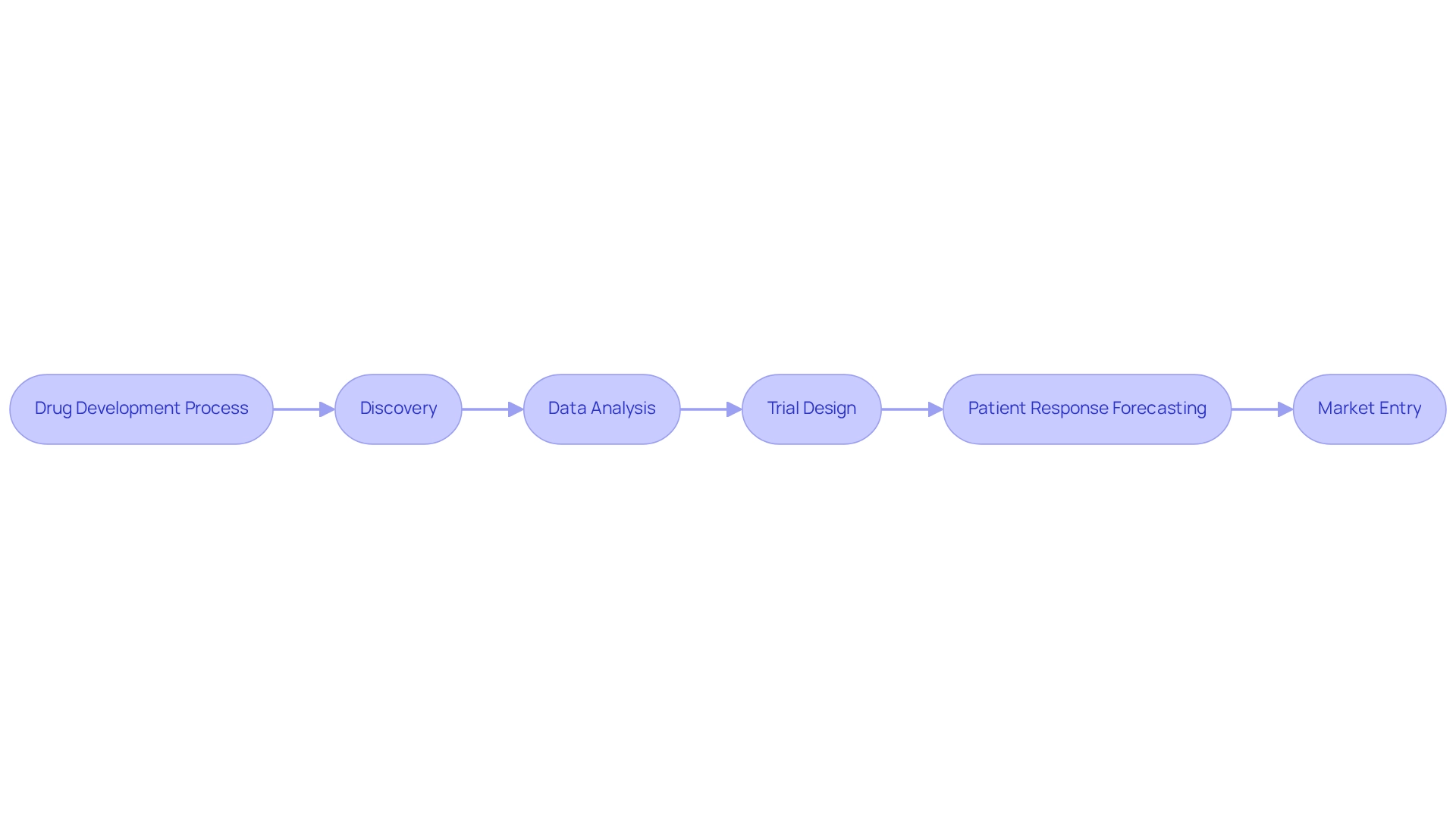
Artificial intelligence (AI) is fundamentally transforming drug development, streamlining processes from discovery to clinical trials. By leveraging advanced algorithms, AI can sift through extensive datasets to pinpoint promising drug candidates, refine trial designs, and forecast patient responses with remarkable accuracy. This not only accelerates time to market but also significantly reduces development costs, enabling the launch of innovative therapies for patients.
In 2025, the incorporation of AI in drug development is anticipated to be crucial for companies aiming to maintain a competitive edge. For instance, AstraZeneca’s application of Model-Informed Drug Development (MIDD) in its respiratory treatments exemplifies how AI can expedite market entry, showcasing the substantial benefits of adopting such technologies. This aligns with CareSet’s insights on identifying new healthcare provider targets and examining prescribing behaviors, illustrating how AI can enhance these processes through innovative Medicare solutions. Statistics indicate that AI is increasingly becoming a cornerstone of drug research, with forecasts suggesting that over 50% of drug development processes will integrate AI-driven methodologies by the year’s end. This encapsulates the broader context of AI’s integration in the drug industry, as evidenced by CareSet’s incorporation of over 100 external information sources.
Insights from technology leaders emphasize the necessity of adopting AI to boost innovation in the drug industry, as it not only enhances efficiency but also fosters a culture of information solidarity—shifting from ‘my information’ to ‘our information’ for collective progress. CareSet’s commitment to information leadership is vital in this transformation, ensuring that stakeholders can collaborate effectively and access comprehensive insights that refine drug strategies and journey mapping for individuals.
As AI advances, its significance in shaping the future of drug development will be essential for companies striving to thrive in a dynamic healthcare landscape. CareSet’s comprehensive strategy not only addresses immediate data needs but also cultivates long-term strategic growth for its partners in the healthcare sector.
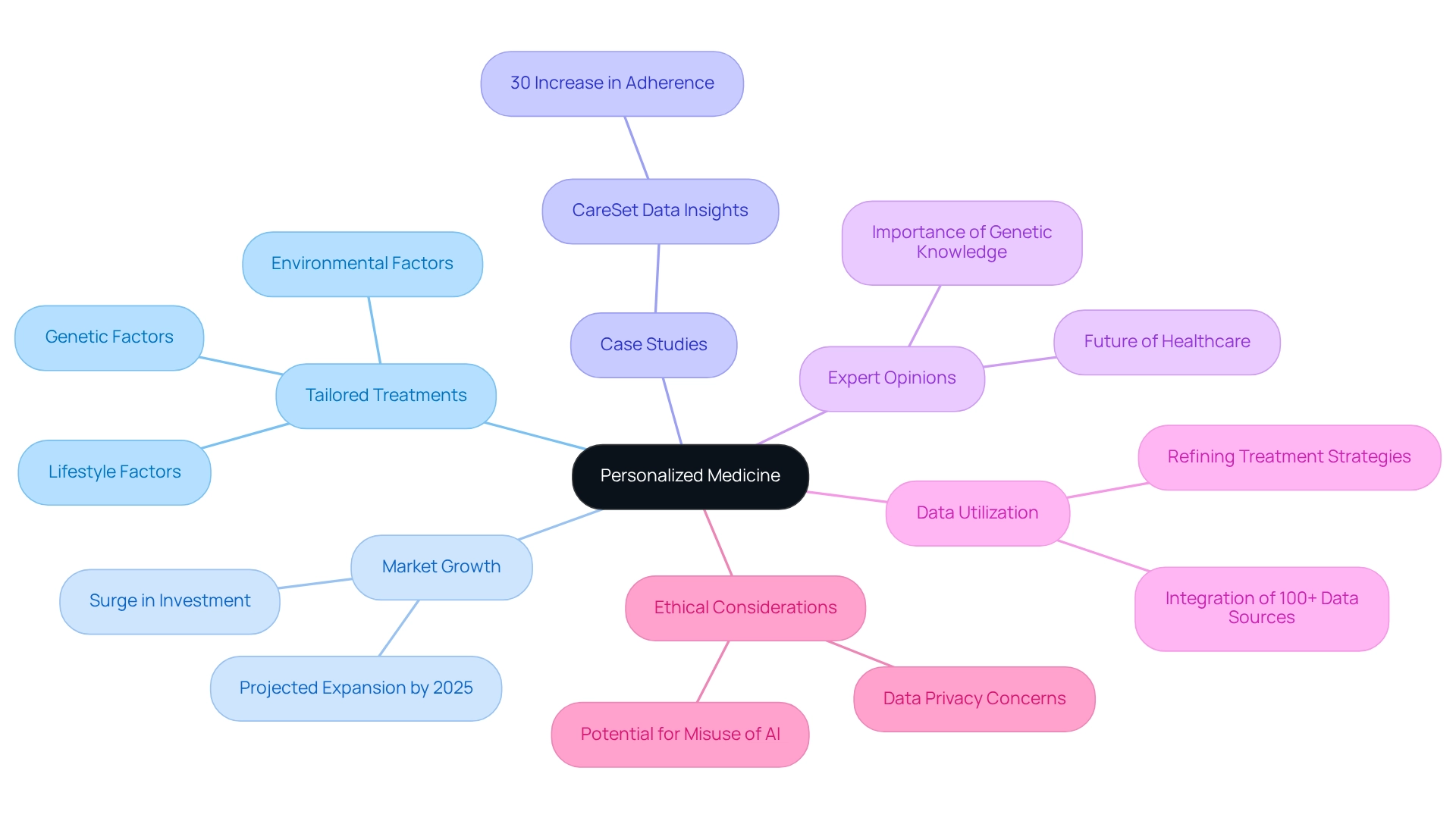
Personalized Medicine: Tailoring Treatments to Individual Patient Needs
Tailored medicine is revolutionizing drug innovation by focusing on the personalization of therapies to meet the specific needs of individuals. By leveraging genetic, environmental, and lifestyle factors, news pharmaceuticals are formulating therapies that not only enhance efficacy but also reduce side effects. This customized approach significantly improves outcomes for patients and fosters greater adherence to treatment plans, as individuals are more likely to engage with therapies designed specifically for their profiles.
As the demand for personalized healthcare intensifies, the medical industry is experiencing a notable surge in investment in news pharmaceuticals aimed at research and development for targeted therapies. By 2025, the personalized medicine market is projected to expand considerably, reflecting a broader shift towards individualized treatment strategies. Successful case studies illustrate how personalized treatments have led to improved adherence and outcomes, underscoring the efficacy of this method. For instance, a recent case study highlighted how a pharmaceutical company utilized CareSet’s data insights to tailor their treatment plans, resulting in a 30% increase in adherence among patients.
Expert opinions emphasize the pivotal role of personalized medicine in shaping the future of healthcare. Geneticists assert that comprehending the genetic foundations of diseases is vital for developing effective treatments, reinforcing the necessity of personalized strategies in contemporary medicine. A geneticist noted, “The integration of genetic knowledge into treatment strategies is not merely beneficial; it is essential for enhancing care for individuals.”
Moreover, CareSet incorporates over 100 external data sources for its analysis, providing a robust framework for understanding treatment patterns and individual demographics. By leveraging CareSet’s comprehensive Medicare data insights, news pharmaceuticals can refine their strategies to address the diverse needs of patient groups, ultimately leading to enhanced healthcare delivery. However, it is also imperative to consider the ethical implications of AI in healthcare, as the potential for misuse raises significant concerns regarding data privacy and the integrity of treatment methodologies.
To explore how CareSet can elevate your personalized medicine strategies, connect with us today and unlock the potential of data-driven insights.
Regulatory Changes: Navigating New Market Access Challenges
Regulatory changes are a constant force in the drug industry, significantly influencing market access strategies for news pharmaceuticals. In 2025, expected regulations are poised to transform pricing, reimbursement, and approval processes in the news pharmaceuticals sector, offering both challenges and opportunities for pharmaceutical firms. Embracing these changes is essential; for instance, the FDA’s Model-Informed Drug Development (MIDD) Pilots aim to establish clear regulatory pathways, fostering proactive dialogue and collaboration within the industry. This initiative is expected to reduce development costs by approximately 20%, equipping companies with the precision and efficiency needed for successful drug development.
To navigate the evolving landscape, companies must adopt a proactive approach that includes continuous monitoring of regulatory developments and adapting strategies accordingly. CareSet’s comprehensive Medicare updates enhance provider engagement by providing novel insights into drug utilization and treatment pathways, addressing urgent information needs while fostering long-term strategic growth for its partners in the healthcare industry. These updates address essential business inquiries related to news pharmaceuticals, including:
- Which diseases providers diagnose and treat
- How individuals move from diagnosis to treatment
- What treatments are being approved by Medicare Part D Plans
By aligning market access initiatives with these regulatory changes in news pharmaceuticals, organizations can ensure compliance and maximize their potential for success in the marketplace. As drug companies adapt to new regulations, they can leverage insights from CareSet’s data solutions, which identify 15% more targets and 250% more individuals than leading claims vendors, demonstrating how strategic adjustments have led to improved market access outcomes. By focusing on both immediate and long-term needs, organizations can position themselves effectively in a competitive environment, ultimately enhancing their market reach and patient engagement.
Sustainability: Emphasizing Eco-Friendly Practices in Pharmaceuticals
Sustainability has emerged as a pivotal focus for the drug industry, with firms increasingly committed to mitigating their environmental impact as highlighted in news pharmaceuticals. This dedication encompasses the adoption of eco-friendly practices across manufacturing, packaging, and distribution processes. By prioritizing sustainability, drug companies not only comply with regulatory requirements but also enhance their brand reputation among consumers who are progressively inclined towards news pharmaceuticals that emphasize environmentally responsible practices.
Research demonstrates that investing in sustainable sourcing can catalyze innovation in eco-friendly products and processes, providing a competitive edge in the market. For instance, GSK’s initiative to source palm oil from RSPO-certified suppliers exemplifies how drug companies can integrate sustainable practices, aligning their operations with broader environmental objectives.
Additionally, effective research and development strategies are essential for advancing sustainability initiatives. A recent case study on the determinants of R&D strategy in sustainability underscores that such strategies significantly enhance productivity and innovation, ultimately improving overall business performance in the healthcare sector, especially in news pharmaceuticals.
As the industry moves towards a greener future, the advantages of implementing sustainable initiatives become increasingly apparent. These practices can result in substantial cost savings, enhanced operational efficiencies, and a stronger market position. Companies that embrace sustainability are not only better equipped to navigate regulatory landscapes but also to cultivate long-term strategic growth.
Insights from industry leaders affirm that these commitments often align with global sustainability goals, such as those outlined by the United Nations, reinforcing the critical role of eco-friendly practices in drug manufacturing. By 2025, the healthcare industry’s emphasis on sustainability will continue to influence market access strategies, driving both innovation and consumer trust.
Telehealth Expansion: Transforming Patient Engagement and Treatment Management
The growth of telehealth services is transforming client engagement and treatment management in the news pharmaceuticals sector. By providing individuals convenient access to healthcare providers, telehealth facilitates timely consultations, follow-ups, and ongoing care management. This evolution not only boosts satisfaction among individuals but also significantly enhances adherence to treatment plans, as they can easily communicate with their healthcare teams. CareSet’s monthly Medicare updates offer essential insights into drug usage and treatment pathways, enabling market access managers in the healthcare sector to enhance their strategies in this dynamic environment.
Emerging trends in telemedicine, such as the use of AI for client analysis and the integration of 5G technology, are expected to further enhance the efficiency and accessibility of telehealth services. As telehealth progresses, companies in the news pharmaceuticals sector must modify their approaches to utilize this technology effectively, ensuring they address the changing requirements of individuals. Furthermore, CareSet’s extensive Medicare information solutions, which examine treatment pathways and provider interventions, can assist in tackling the difficulties healthcare providers encounter in integration across devices and users.
With the number of individuals aged 65 and older expected to rise by 42.4 percent by 2034, the need for accessible healthcare solutions will only grow, making telehealth a crucial part of future medical strategies. To leverage telehealth effectively, market access managers in news pharmaceuticals should focus on integrating robust data systems that facilitate seamless communication and enhance patient engagement.
Mental Health Focus: Increasing Investment in Therapeutic Solutions
The drug industry is undergoing a significant transformation, increasingly focusing on mental health. This shift is characterized by a notable rise in investments aimed at therapeutic solutions to address the growing prevalence of mental health disorders. As awareness of these issues deepens, pharmaceutical companies are prioritizing the development of innovative treatments tailored for this critical sector. This strategic pivot not only addresses the urgent needs of patients but also unveils new market opportunities for drug firms. By channeling resources into mental health solutions, organizations can enhance their product offerings while contributing to improved public health outcomes.
Investment trends reveal a cautious yet optimistic strategy, with funding for AI-driven mental health initiatives dipping from 53% in 2023 to 48% in 2024. This change indicates a growing inclination among investors toward solutions that exhibit immediate clinical validation and regulatory approval, highlighting the significance of established therapeutic models. Moreover, the increased focus on mental health within the drug industry is expected to stimulate innovation, leading to the creation of new therapeutic solutions designed to address a spectrum of mental health disorders.
As the market evolves, drug companies are urged to adapt their strategies in response to these trends, especially in light of the recent decrease in H2 partnership activity. This dynamic environment necessitates a proactive stance to maintain competitiveness in a market that increasingly values mental health initiatives. Additionally, over half (55%) of insurers in the MEA and the Americas cite advancements in medications as a pivotal factor influencing medical expenses, underscoring the broader implications of these investment trends. Companies should consider leveraging CareSet’s extensive healthcare data insights to align their product development with these investment patterns, concentrating on innovative solutions that fulfill both patient needs and market demands. By utilizing data-driven insights, drug companies can make informed investment decisions that bolster their competitiveness in the evolving mental health landscape.
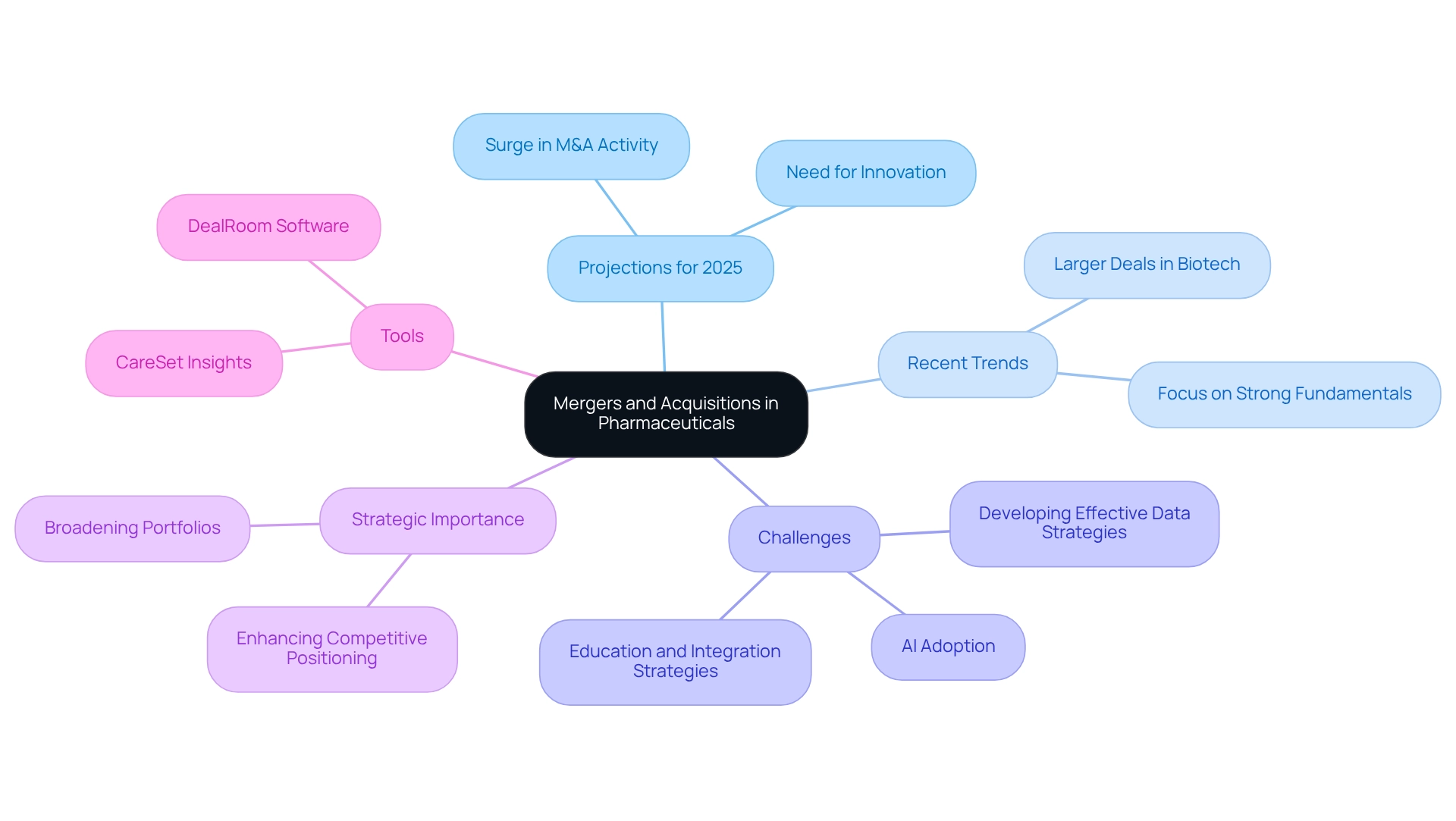
Mergers and Acquisitions: Reshaping the Pharmaceutical Landscape
Mergers and acquisitions (M&A) are fundamentally transforming the healthcare landscape as firms seek to enhance their competitive positioning and broaden their portfolios. Projections for 2025 indicate a significant surge in M&A activity, driven by the urgent need for innovation and the pursuit of strategic partnerships. By acquiring complementary assets or technologies, drug companies can accelerate their research and development initiatives, improve market access, and strengthen their overall value proposition.
Recent trends reveal a shift towards fewer but larger deals, with the U.S. and European biotech sectors raising $23.36 billion in 2024. This underscores a focus on biotechs that demonstrate robust business fundamentals and clear exit strategies. Such a trend highlights the critical importance of both innovation and execution in successful M&A efforts. Moreover, industry specialists emphasize that efficient information strategies, the adoption of artificial intelligence, and comprehensive education and integration approaches are essential for fostering successful collaborations with technology firms. The industry faces challenges in developing these strategies, which are vital for navigating the complexities of M&A. As KPMG notes, healthcare and life sciences leaders are optimistic about an increase in M&A activity in 2025, emphasizing the necessity of understanding these dynamics.
In this context, CareSet’s extensive healthcare information insights can play a pivotal role in supporting drug companies during M&A activities. By leveraging actionable data, stakeholders can make informed decisions that enhance their strategic positioning and optimize their integration processes. As stakeholders navigate this evolving landscape, grasping the intricacies of M&A in news pharmaceuticals becomes essential for seizing emerging opportunities and driving pharmaceutical innovation. Furthermore, platforms like DealRoom are recognized as leading M&A software solutions that can bolster these strategic initiatives.
Conclusion
The pharmaceutical industry is currently experiencing a significant transformation, marked by innovative strategies that leverage data-driven insights and advanced technology. By utilizing Medicare data, companies can enhance their market access strategies and optimize patient engagement, which in turn leads to improved healthcare outcomes. The transition towards value-based pricing underscores the necessity of aligning drug costs with patient results, ensuring that treatments provide measurable benefits.
Artificial intelligence is expediting drug development, facilitating faster and more cost-effective processes, while personalized medicine is customizing treatments to meet individual patient needs, thus improving adherence and outcomes. Regulatory changes are reshaping the industry landscape, necessitating that companies adapt proactively to navigate these challenges effectively. Concurrently, sustainability and mental health initiatives are gaining traction, reflecting a broader commitment to responsible practices and patient well-being.
As mergers and acquisitions redefine the competitive landscape, comprehending these dynamics becomes essential for stakeholders aiming to excel in this evolving ecosystem. The integration of data insights, innovative pricing models, and a focus on patient-centric solutions will be crucial for pharmaceutical companies striving not only to survive but to thrive in this complex environment. Embracing these multifaceted strategies will ultimately foster long-term growth and enhance the effectiveness of market access initiatives, positioning companies for success in the future of healthcare.

