Overview
The primary objective of the article “9 Essential Process Measure Strategies for Pharmaceutical Success” is to delineate critical strategies that pharmaceutical companies can adopt to bolster their operational efficiency and market performance. It underscores the significance of leveraging data-driven insights, including Medicare claims analysis and methodologies such as the Balanced Scorecard, to guide decision-making processes. By optimizing resource allocation, these strategies aim to enhance patient outcomes and improve market access. Ultimately, this approach not only fosters a more effective operational framework but also positions companies to respond adeptly to the evolving landscape of the pharmaceutical industry.
Introduction
In the rapidly evolving pharmaceutical landscape, data-driven insights have become indispensable for navigating complexities and enhancing patient care. Companies are striving to optimize their operations and improve market access strategies, with innovative methodologies such as:
- Medicare data analysis
- The Balanced Scorecard
- Process mining
leading this transformation. By harnessing comprehensive metrics and analytics, organizations can identify inefficiencies, monitor compliance, and ultimately drive better health outcomes. This article explores the critical frameworks and tools that empower pharmaceutical companies to refine their strategies, ensuring they remain competitive while delivering value to patients and stakeholders alike.
CareSet: Comprehensive Medicare Data Analysis for Process Measurement
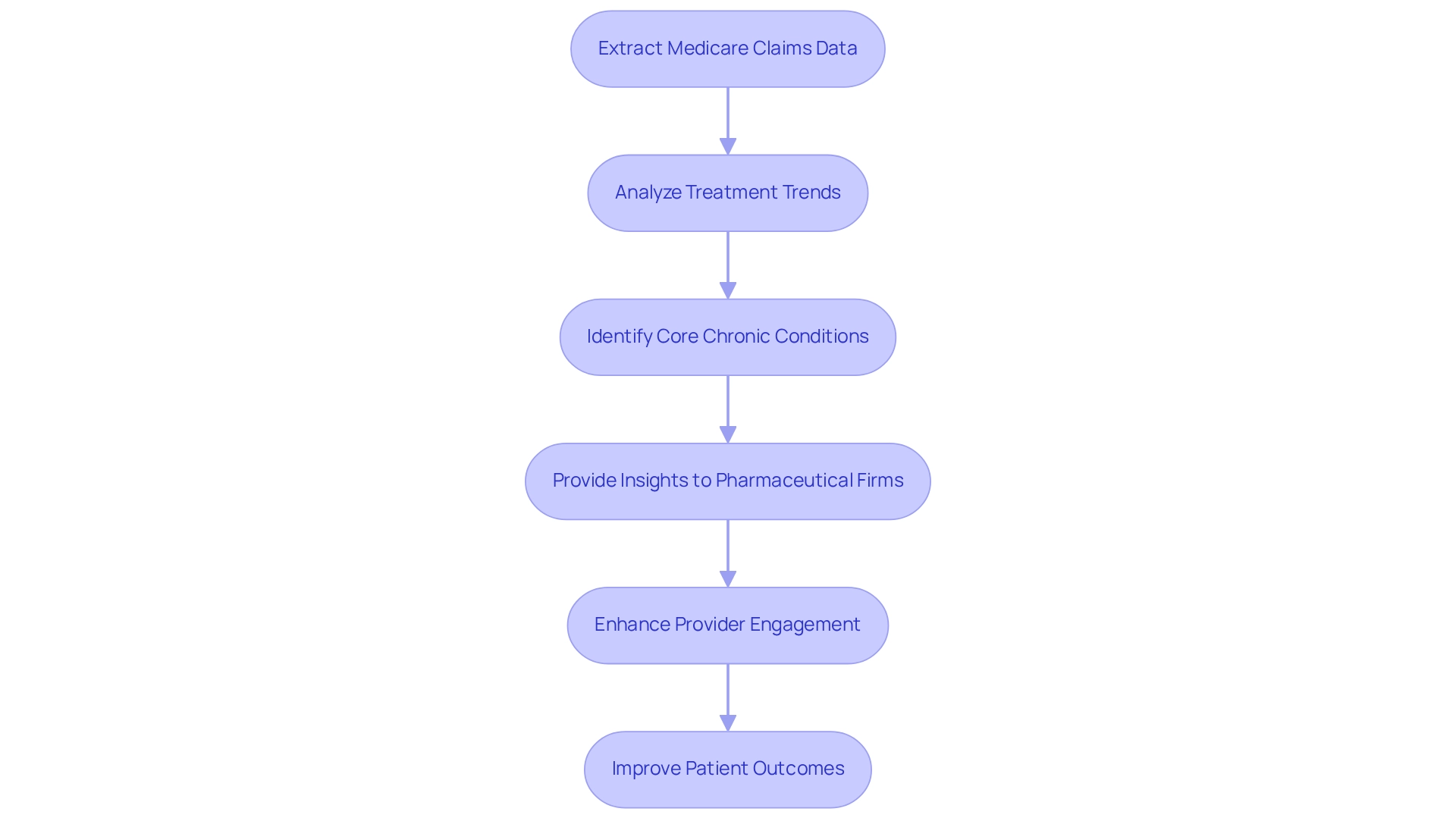
CareSet excels in extracting and interpreting intricate Medicare claims information, providing drug companies with essential insights into treatment trends and demographic details. By examining over $1.1 trillion in yearly claims data, CareSet enables stakeholders to pinpoint information gaps and effectively chart individual journeys, which are crucial for accurate process measure in the pharmaceutical industry. This data-driven approach not only supports informed decision-making but also significantly enhances care and operational efficiency.
The influence of Medicare data on healthcare insights is profound, as it facilitates the identification of core chronic conditions targeted by Medication Therapy Management (MTM) programs, including:
- Alzheimer’s
- diabetes
- hypertension
- respiratory diseases
Such insights are essential for pharmaceutical firms seeking to refine their market access strategies and boost client engagement.
For instance, in a recent case study involving an oncology treatment manufacturer, CareSet’s insights enabled the company to enhance its engagement with healthcare providers regarding the 4th line of therapy for Gastrointestinal Stromal Tumor (GIST). This case exemplifies how utilizing Medicare information can lead to more effective treatment options and improved patient outcomes.
Recent developments, such as Avalere’s expedited access to Medicare information through the Virtual Data Research Center, underscore the increasing significance of timely insights in navigating the evolving healthcare landscape. Maddi Davidson, Principal at Avalere, stated, “This new access to CMS information enables Avalere to speed up our capacity to offer clients insights more quickly.” This access empowers organizations to conduct thorough healthcare journey studies and respond efficiently to urgent health inquiries, especially in light of recent policy modifications and the ongoing effects of COVID-19.
In 2025, the administrative expenses related to the Mid-Year Notification of Unused Supplemental Benefits highlight the financial implications of Medicare information management, totaling $23.7 million. This statistic underscores the necessity for drug manufacturers to leverage precise Medicare claims information to optimize their operational strategies and enhance health outcomes. Understanding these expenses can assist organizations in more effectively allocating resources and improving their overall efficiency in managing Medicare data.
Ultimately, CareSet’s commitment to delivering high-quality, comprehensive Medicare insights positions its clients for success in a competitive market, ensuring they can navigate the complexities of healthcare with confidence and clarity. By offering actionable insights tailored to the challenges faced by drug firms, CareSet empowers its clients to make informed decisions that enhance patient care and operational efficiency.
The Balanced Scorecard: A Framework for Measuring Pharmaceutical Performance
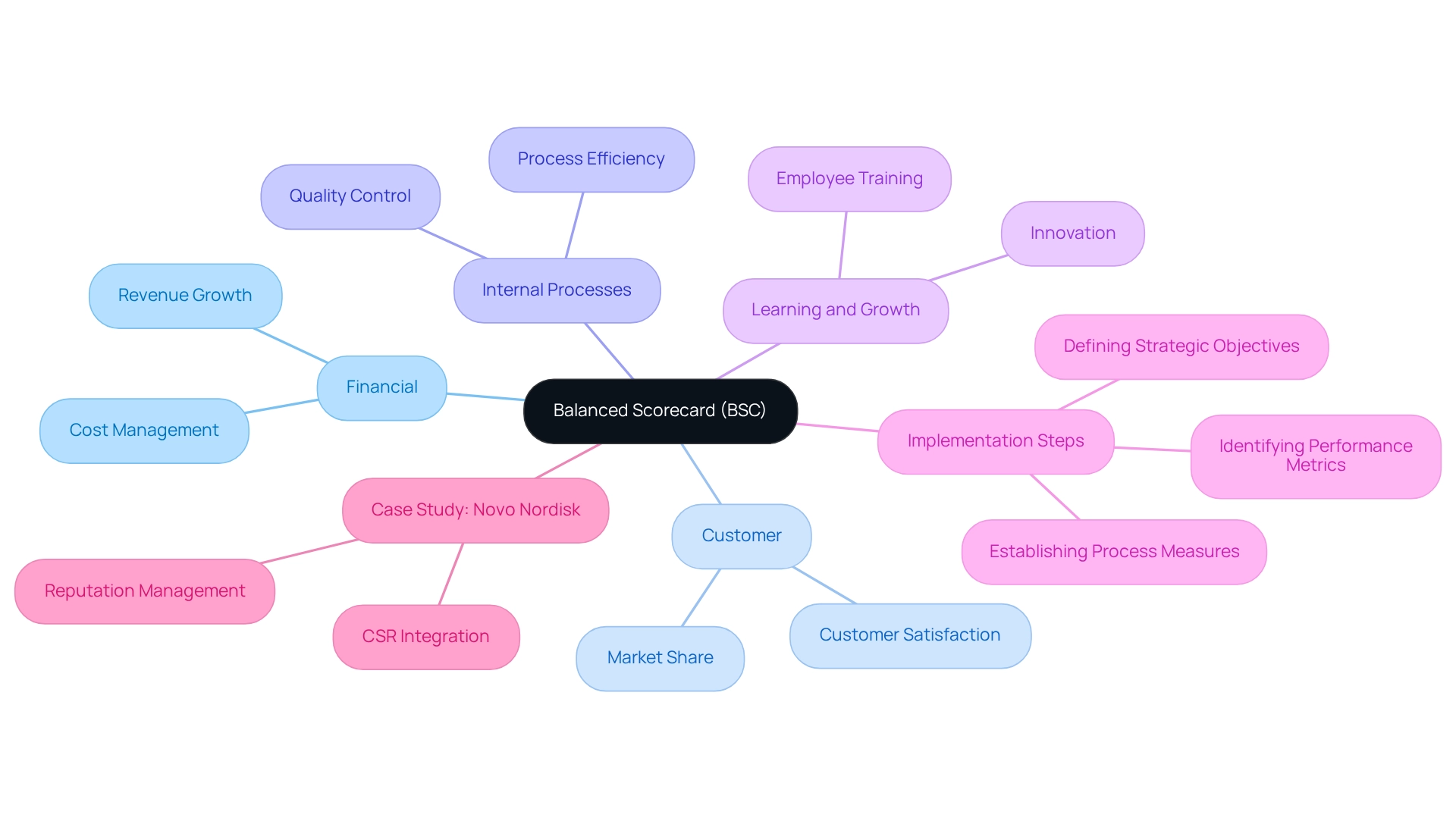
The Balanced Scorecard (BSC) serves as a transformative framework for drug manufacturers, enabling them to translate strategic goals into a comprehensive set of process measures for performance. By encompassing four critical perspectives—financial, customer, internal processes, and learning and growth—the BSC provides a holistic view of organizational performance. This multifaceted approach not only assists in tracking progress but also aligns daily operations with overarching strategic objectives, thereby improving decision-making and driving better results.
As of 2025, the adoption of the Balanced Scorecard in the drug industry has gained significant momentum, with current statistics indicating that over 60% of top organizations utilize this framework to enhance performance measurement. Implementing the BSC involves several key steps:
- Defining strategic objectives
- Identifying relevant performance metrics
- Establishing a process measure for regular review and adjustment
This structured method ensures that businesses remain agile and responsive to market changes.
A notable example of successful BSC implementation can be observed in organizations like Novo Nordisk, which has effectively utilized the framework to align its operations with its commitment to corporate social responsibility (CSR). The case study of Novo Nordisk illustrates how the BSC not only aids in performance assessment but also strengthens the organization’s ethical position in a competitive market by incorporating CSR objectives into their strategic aims. The effectiveness of the Balanced Scorecard in evaluating industry performance is underscored by its capacity to deliver actionable insights that propel strategic initiatives. By integrating data from various sources, including CareSet’s innovative data science products and access to over 62 million Medicare beneficiaries and 6 million providers, companies can gain a comprehensive understanding of their performance landscape. This data-driven approach empowers healthcare organizations to make informed decisions that enhance patient care and optimize product lifecycle management, ultimately supporting their drug launch strategies and overall healthcare insights.
Statistical Process Control (SPC): Ensuring Quality in Pharmaceutical Processes
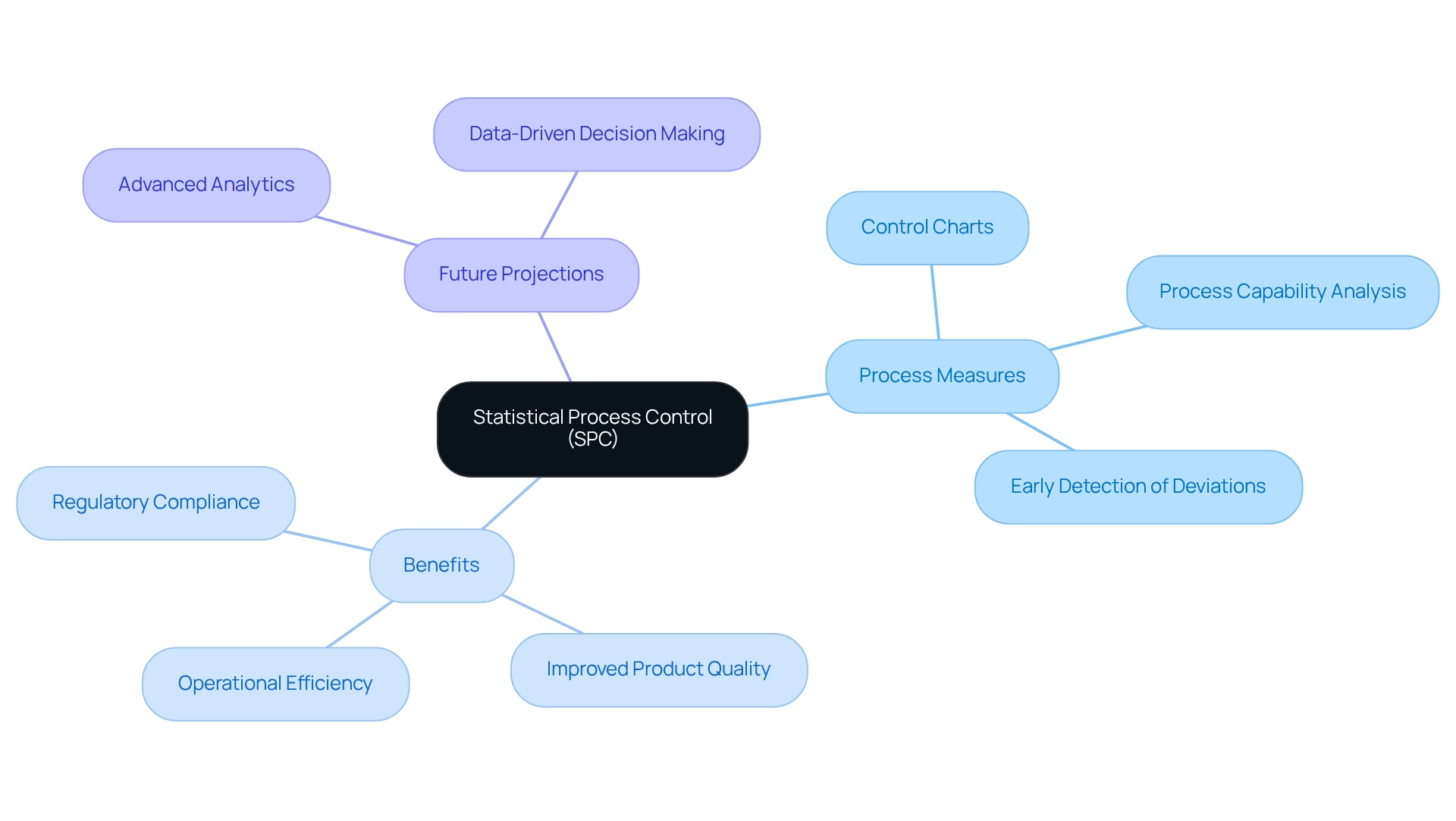
Statistical Process Control (SPC) serves as a crucial process measure in the drug manufacturing sector, overseeing and regulating production processes through statistical methods. SPC acts as a process measure by employing control charts and process capability analysis, enabling drug manufacturers to detect variations that could potentially lead to quality issues. Projections for 2025 indicate that the adoption of SPC will significantly enhance compliance with regulatory standards, improve product quality, and elevate operational efficiency. This proactive approach not only mitigates risks associated with manufacturing inconsistencies but also contributes to improved patient outcomes, highlighting the process measure of SPC in quality assurance within drug manufacturing. It allows for the early detection of deviations, facilitating timely interventions that prevent defects and ensure that products adhere to stringent quality criteria. Moreover, SPC cultivates a culture of continuous improvement by utilizing process measures to empower organizations to systematically refine their processes. As pharmaceutical companies increasingly acknowledge the importance of data-driven decision-making, integrating SPC into their quality assurance frameworks becomes essential for maintaining competitive advantage and ensuring the safety and efficacy of their products.
In 2025, the anticipated impact of SPC on pharmaceutical quality assurance is expected to be significant, with companies leveraging advanced analytics to enhance their monitoring capabilities. This evolution in quality management addresses urgent information needs while supporting long-term strategic growth, positioning organizations to adeptly navigate the complexities of the healthcare landscape. For instance, practices related to cookie duration in information management, such as Epsilon’s extensive data collection with prolonged cookie durations and Ensighten’s 1825-day cookie duration, underscore the evolving landscape of industry practices. These examples highlight the importance of comprehensive data management strategies that SPC can support, ensuring drug manufacturers are well-equipped to tackle both current and future challenges.
Process Mining: Uncovering Inefficiencies in Pharmaceutical Operations
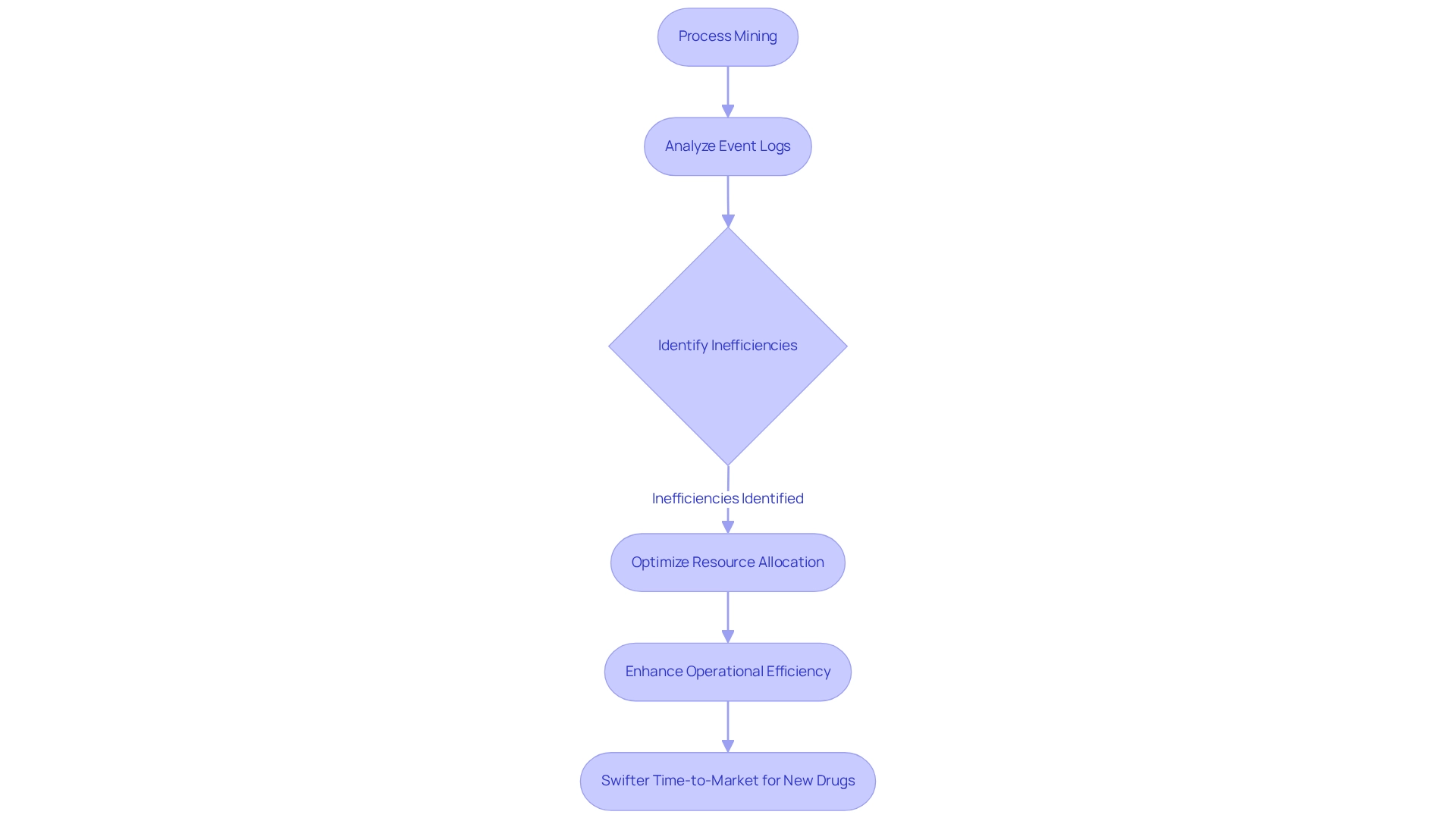
Process mining stands as a formidable analytical technique that uses process measures to scrutinize event logs, unveiling, monitoring, and refining real-world processes. Within the drug industry, this methodology is indispensable for identifying inefficiencies in workflows, such as production bottlenecks and delays in regulatory compliance. By visualizing the actual flow of operations, organizations can pinpoint critical areas for enhancement, optimize resource allocation, and markedly elevate operational efficiency. This results in a swifter time-to-market for new drugs, a vital factor in a fiercely competitive landscape.
CareSet Systems’ innovative data science solutions, particularly their extensive Medicare data offerings, empower drug manufacturers by providing insights derived from over 62 million beneficiaries and 6 million providers. These insights can be harnessed to refine drug launch strategies and enhance overall healthcare outcomes. For example, organizations leveraging CareSet’s data-driven insights in conjunction with process measures can uncover inefficiencies that traditional methods might miss. By employing advanced techniques, they can streamline workflows, reduce cycle times, and bolster compliance with regulatory standards.
The process mining market is poised for significant growth, indicating that organizations embracing this technology, particularly those utilizing CareSet’s solutions, will gain a competitive edge. A compelling case study is Dabur’s ongoing transformation journey, which seeks to embed long-term value for its stakeholders. Their strategic initiatives, bolstered by process mining and CareSet’s insights, are anticipated to enhance stakeholder engagement and drive sustainable growth, exemplifying how these tools can facilitate substantial improvements in operational practices.
Current statistics reveal that organizations utilizing process measures in conjunction with process mining techniques in drug-related operations, especially those guided by CareSet’s Medicare insights, are witnessing noteworthy enhancements in operational efficiency. As we advance through 2025, the impact of these techniques will become increasingly evident, with organizations that adopt them likely to experience heightened productivity and reduced operational costs. In conclusion, process mining serves as a process measure that uncovers inefficiencies and acts as a catalyst for operational excellence in the healthcare sector, particularly when integrated with CareSet’s comprehensive data solutions. As Arnold H. Glasow aptly stated, “Improvement begins with I,” underscoring the personal responsibility in driving enhancements through process mining.
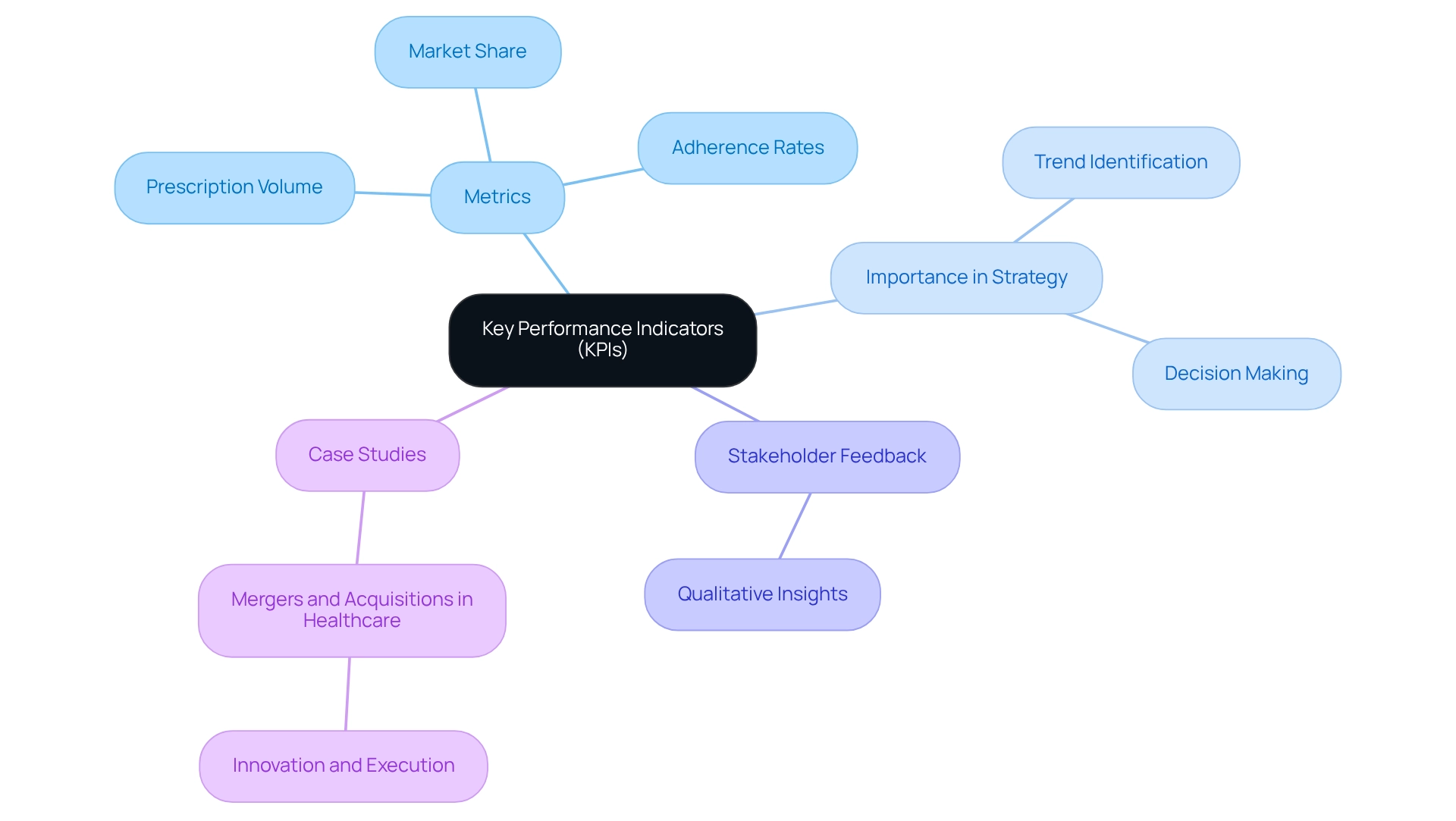
Key Performance Indicators (KPIs): Measuring Success in Market Access Strategies
Key Performance Indicators (KPIs) are essential quantifiable process measures that empower pharmaceutical companies to assess the effectiveness of their market access strategies. Metrics such as prescription volume, market share, and adherence rates are critical in this evaluation. For instance, in 2025, the monitoring of prescription volume and market share will be pivotal as the industry anticipates shifts in demographic groups and treatment patterns.
By consistently tracking these KPIs as a process measure, companies can identify trends and make informed adjustments to their strategies, ultimately enhancing market presence and client engagement. As Jermaine Jackson, a managing partner, notes, “In the healthcare sector, Key Performance Indicators (KPIs) are the compass directing business strategies and individual well-being.” This statement underscores the necessity of a robust set of KPIs that, when effectively communicated, reveal the pulse of success in both health outcomes and business objectives.
Furthermore, gathering qualitative feedback from various stakeholders acts as a vital process measure that enriches the understanding of a product’s effectiveness, aligning with the ultimate aim of KPIs: to drive better decision-making and improve patient outcomes. Successful KPI tracking is exemplified in case studies analyzing mergers and acquisitions in healthcare, where fewer but larger deals have highlighted the importance of innovation and execution in enhancing competitive positioning. These case studies demonstrate how effective KPI tracking serves as a process measure that influences strategic decisions, showcasing the tangible impact of these metrics.
As the healthcare landscape evolves, the commitment to high-quality information insights, such as those provided by CareSet, which integrates over 100 external information sources, will be crucial for organizations navigating complexities and fostering long-term strategic growth. CareSet’s Medicare data solutions empower drug manufacturers to leverage actionable insights for market access strategies. By focusing on current KPIs for market access strategies, drug manufacturers can position themselves for success in 2025 and beyond. Additionally, as Vanessa Burrow, a consultant, emphasizes, “There is a clear disconnect between accepted marketing measurement theory and the definition and use of KPIs and metrics in practice,” highlighting the challenges that must be addressed to fully harness KPIs in the industry.
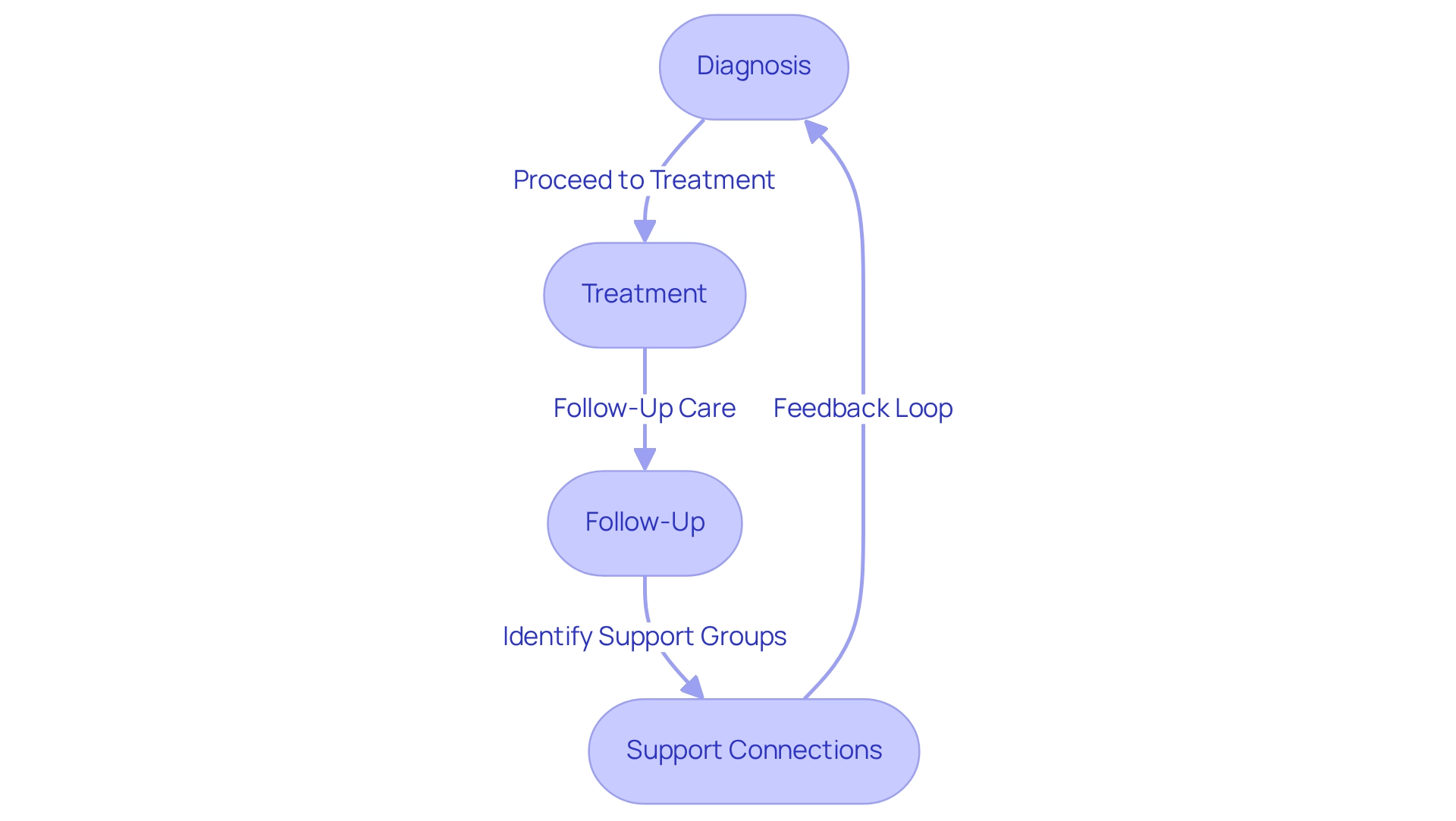
Patient Journey Mapping: Tracking Patient Interactions for Better Outcomes
Journey mapping serves as a vital strategic tool that visualizes the comprehensive steps an individual undertakes throughout their healthcare process measure. By carefully mapping interactions from diagnosis through treatment and follow-up, drug manufacturers can reveal essential insights into individual needs and preferences. This nuanced understanding enables the development of targeted interventions that not only enhance client satisfaction but also significantly improve treatment outcomes, highlighting the role of process measures.
Current statistics indicate that effective journey mapping as a process measure can lead to a notable increase in treatment adherence, with studies showing that organizations implementing these strategies report up to a 30% improvement in compliance. Moreover, expert views emphasize the significance of care focused on individuals, pointing out that when individuals feel comprehended and supported, their chances of following prescribed regimens rise significantly.
CareSet’s innovative Medicare data solutions play a vital role in this process measure by providing thorough insights into individual journeys across Medicare Parts A, B, and D. By utilizing data from over 62 million beneficiaries and 6 million providers, CareSet enables drug manufacturers to analyze treatment pathways and provider interventions effectively. For example, comprehending how individuals move from diagnosis to treatment through the use of ICD, NDC, and HCPCS codes enables a more customized approach to care.
Additionally, many individuals remain unaware of available support groups for their conditions, which can obstruct their treatment journey. By incorporating journey mapping of individuals as a process measure into their strategies, drug manufacturers can pinpoint these gaps and promote connections, ultimately cultivating a more supportive atmosphere for individuals. This is especially significant as the financial consequences of excessive testing can weigh heavily on individuals, making it vital for organizations to comprehend and address individual needs efficiently.
In summary, mapping the journey of individuals not only clarifies the complexities of interactions but also acts as a foundation for creating effective strategies that boost treatment adherence and enhance overall outcomes in the healthcare sector. To commence executing journey mapping for individuals, drug companies should initiate by collecting information on interactions and feedback from patients, enabling them to recognize key touchpoints and areas for enhancement.
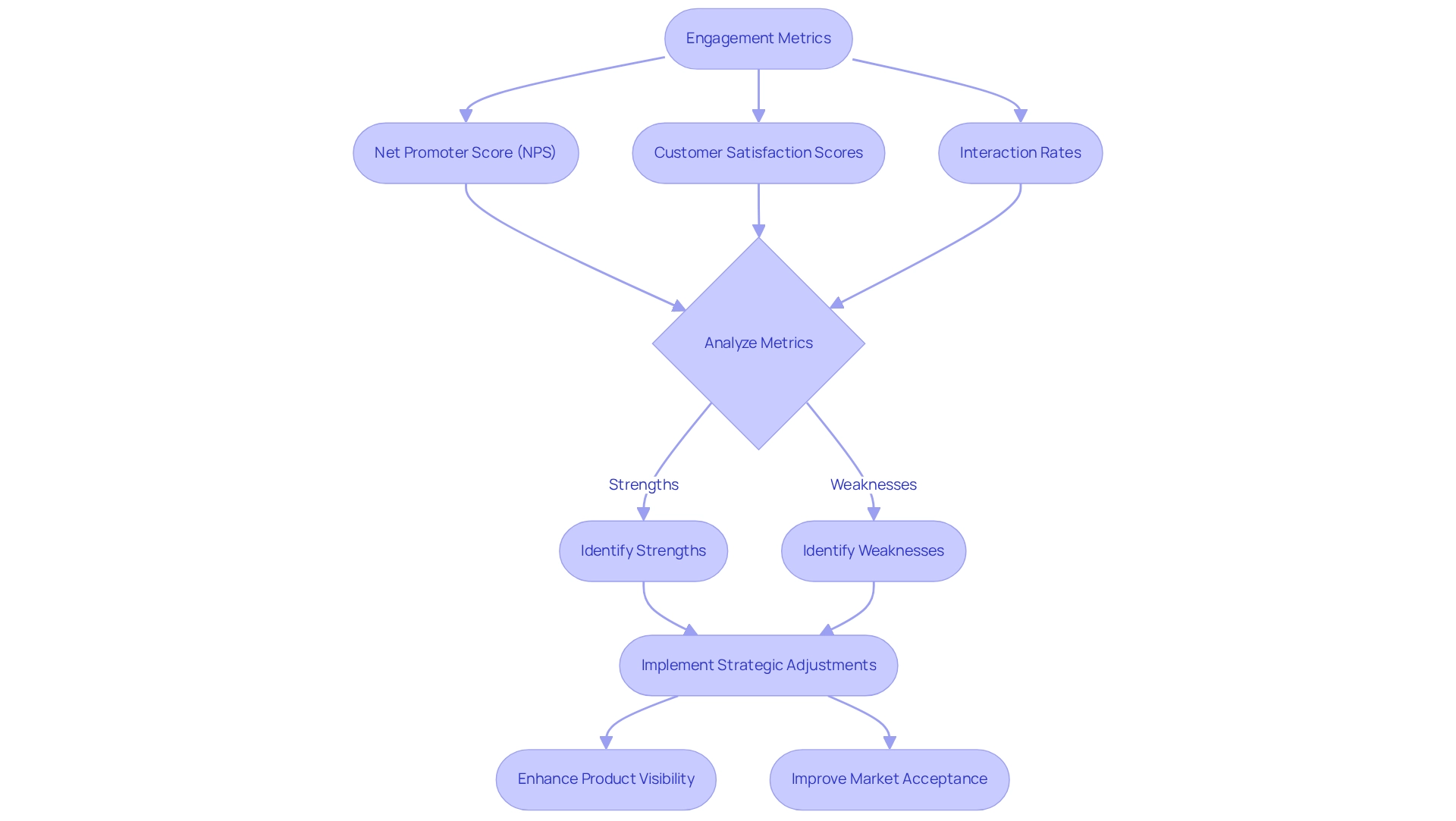
Engagement Metrics: Evaluating Pharmaceutical Product Resonance
Engagement metrics, including Net Promoter Score (NPS), customer satisfaction scores, and interaction rates, are essential for evaluating the connection between products and healthcare professionals as well as individuals. Notably, in 2025, the medicine sector has undergone a significant transformation, with patients now three times more inclined to utilize search methods—such as online research and digital platforms—over traditional means like referrals or direct inquiries to find hospitals. This shift underscores the importance of implementing effective digital engagement strategies, where process measures allow organizations to meticulously analyze these metrics and pinpoint both strengths and weaknesses in their marketing approaches. This data-driven insight facilitates strategic adjustments that enhance product visibility and market acceptance. For example, the successful implementation of NPS has emerged as a valuable tool for gauging customer loyalty and satisfaction, offering actionable feedback that can drive improvements in product offerings. Industry insights reveal that organizations effectively leveraging NPS can witness notable growth in customer retention and advocacy.
Furthermore, case studies focusing on oncology treatment options highlight the critical role of engagement metrics in healthcare marketing. Understanding treatment pathways and provider interventions through Medicare data empowers healthcare stakeholders to make informed decisions regarding care. The evaluation of payment policies and new technologies under Medicare incentivizes hospitals to adopt innovations that demonstrate substantial clinical improvement. Yet, challenges in coding and billing frequently obstruct hospitals from receiving full payments, indicating an urgent need for enhanced systems that align more closely with engagement metrics. This relationship illustrates how engagement metrics, as a process measure, can inform strategic decisions in the realm of healthcare reimbursement. As the landscape evolves, comprehending and utilizing this process measure will be vital for companies aiming to optimize their market strategies and enhance patient outcomes. The focus on these metrics not only aids in assessing product resonance but also fosters long-term relationships with healthcare providers, ultimately driving success in the competitive pharmaceutical market. As noted by Howard P. Forman, M.D., strengthening the connection between evidence and insurance coverage is crucial for informed decision-making, further highlighting the significance of robust data in evaluating product resonance.
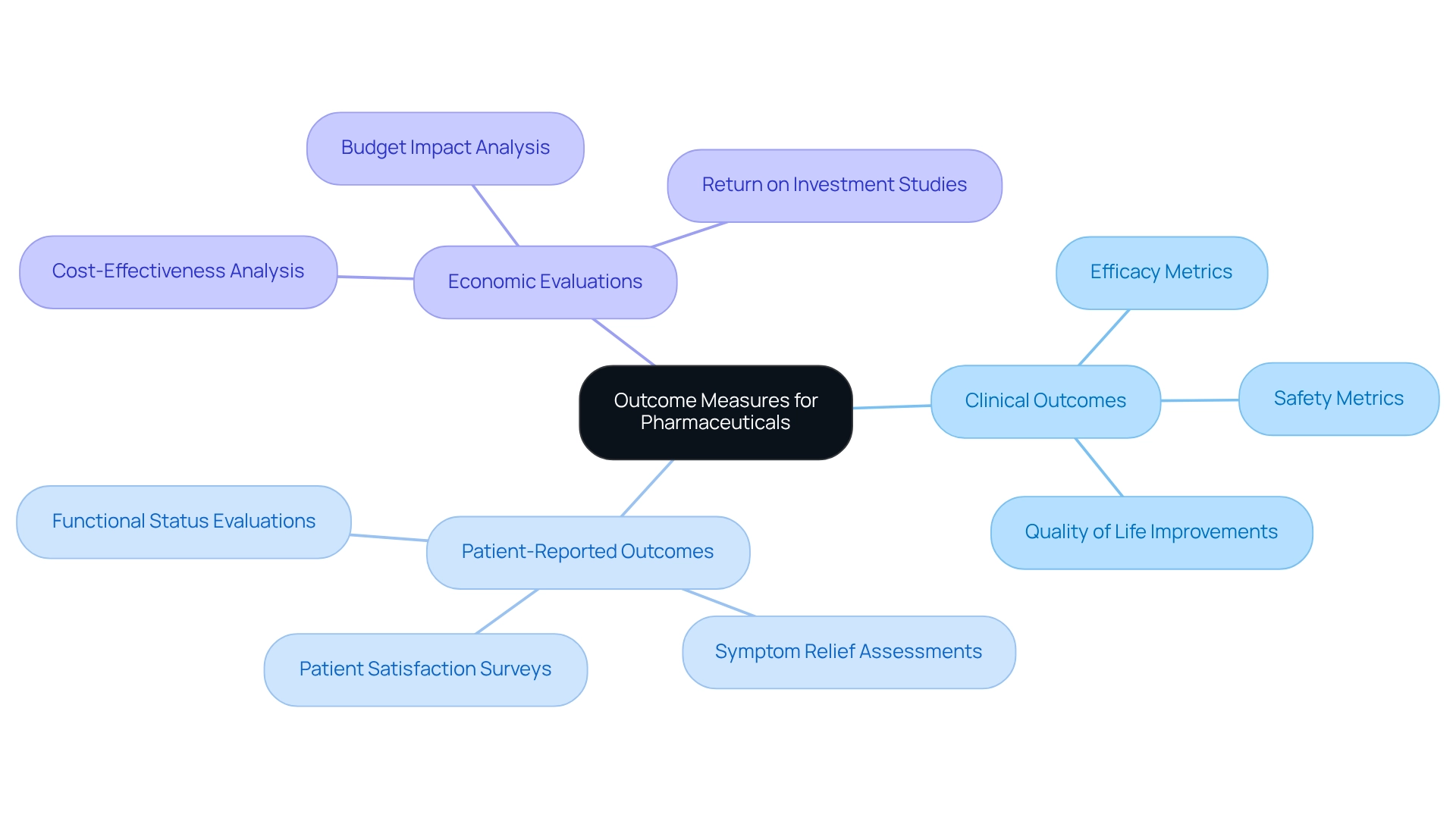
Outcome Measures: Demonstrating Value of Pharmaceutical Interventions
Process measures serve as essential metrics in evaluating the effectiveness of medical interventions on patient health and overall quality of life. These process measures include clinical outcomes, patient-reported outcomes, and economic evaluations, providing a comprehensive perspective on a drug’s impact. As we approach 2025, the emphasis on demonstrating the value of pharmaceuticals through positive process measures is more pronounced than ever. Stakeholders increasingly demand robust evidence of efficacy and cost-effectiveness.
Pharmaceutical companies that adeptly showcase their products through strong outcome measures can significantly bolster their credibility with payers and healthcare providers. This credibility is crucial for enhancing market access and securing favorable reimbursement opportunities. Notably, the biologics market is projected to experience rapid expansion, with a compound annual growth rate of 15% anticipated until 2027. Companies prioritizing process measures will not only address current market demands but will also position themselves for long-term success in an increasingly competitive landscape.
Case studies vividly illustrate the tangible benefits of implementing process measures. For instance, CareSet’s Medicare information analysis demonstrates how actionable intelligence derived from comprehensive insights into treatment patterns can lead to improved treatment analytics and market reach. A noteworthy example involves an oncology treatment manufacturer that leveraged CareSet’s Medicare data to enhance engagement with healthcare providers concerning the 4th line of therapy, Qinlock, for Gastrointestinal Stromal Tumor (GIST). By employing these insights, the organization effectively communicated the value of their treatment options, ultimately improving patient outcomes and expanding their market presence, with process measures that validate the effectiveness of drugs and enhance market access, rendering them indispensable tools for industry success.
As the demand for evidence-based outcomes continues to rise, utilizing CareSet’s insights will be critical for drug manufacturers aiming to adeptly navigate the complexities of the healthcare landscape.
Compliance Metrics: Ensuring Regulatory Adherence in Pharmaceuticals
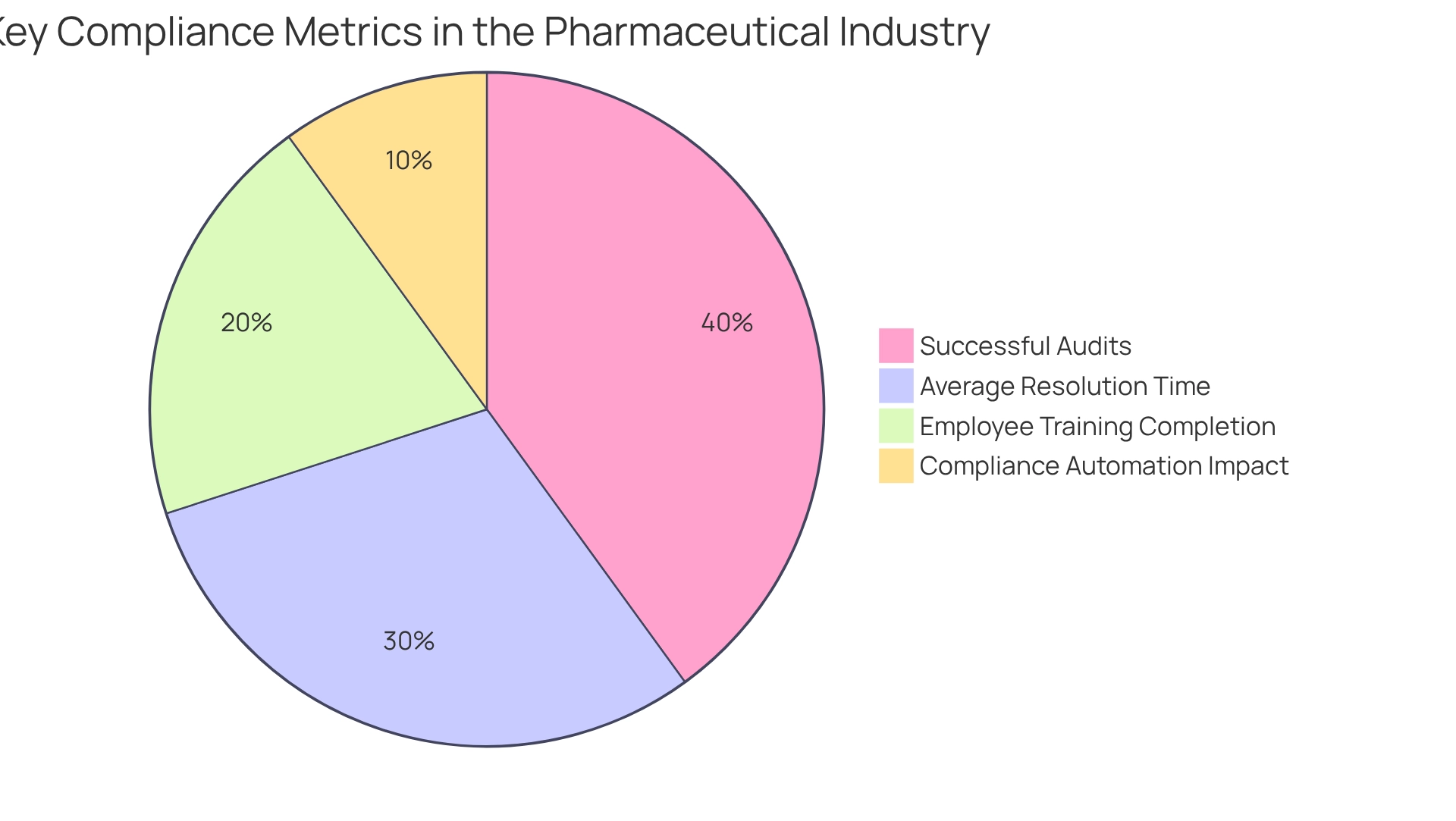
Compliance metrics serve as critical indicators of a pharmaceutical firm’s adherence to regulatory standards. These quantifiable measures encompass the frequency of compliance violations, the duration required to address audit findings, and the efficacy of corrective actions implemented.
In 2025, the landscape of regulatory adherence is increasingly shaped by automation, with a notable shift towards compliance technologies that streamline monitoring processes. Statistics reveal that 53% of businesses still leave sensitive data files accessible to unauthorized personnel, underscoring the urgent need for robust compliance frameworks; to effectively monitor regulatory adherence, organizations should establish process measures alongside a set of key performance indicators (KPIs) tailored to their specific operations. These KPIs can include metrics such as:
- The percentage of successful audits
- The average time taken to resolve compliance issues
- The rate of employee training completion on compliance protocols
By regularly assessing these metrics as a process measure, pharmaceutical firms can not only ensure compliance but also enhance product quality and maintain public trust. As noted by Gurudev Mallesha, ‘Compliance becomes manageable when organizations start early.’ Early integration of compliance practices can significantly ease the burden of regulatory adherence as companies scale.
Case studies illustrate that organizations adhering to international standards, such as PCI DSS and ISO 27001, have avoided substantial financial penalties and improved their data security posture. In fact, businesses that conduct regular compliance audits save an average of $2.86 million, highlighting the financial benefits of maintaining rigorous compliance metrics. Furthermore, with 173 countries having ISO members representing them, the importance of international compliance standards cannot be overstated.
In the realm of the healthcare sector, it is crucial to recognize that 90% of compliance professionals find GDPR compliance the most challenging to achieve. This statistic underscores the complexities faced in regulatory adherence, particularly in this sector.
In summary, the current compliance metrics environment in the drug industry emphasizes the importance of continuous monitoring and improvement through process measures. By concentrating on these metrics, organizations can not only satisfy regulatory requirements but also promote quality improvements and encourage long-term success in an increasingly intricate regulatory landscape.
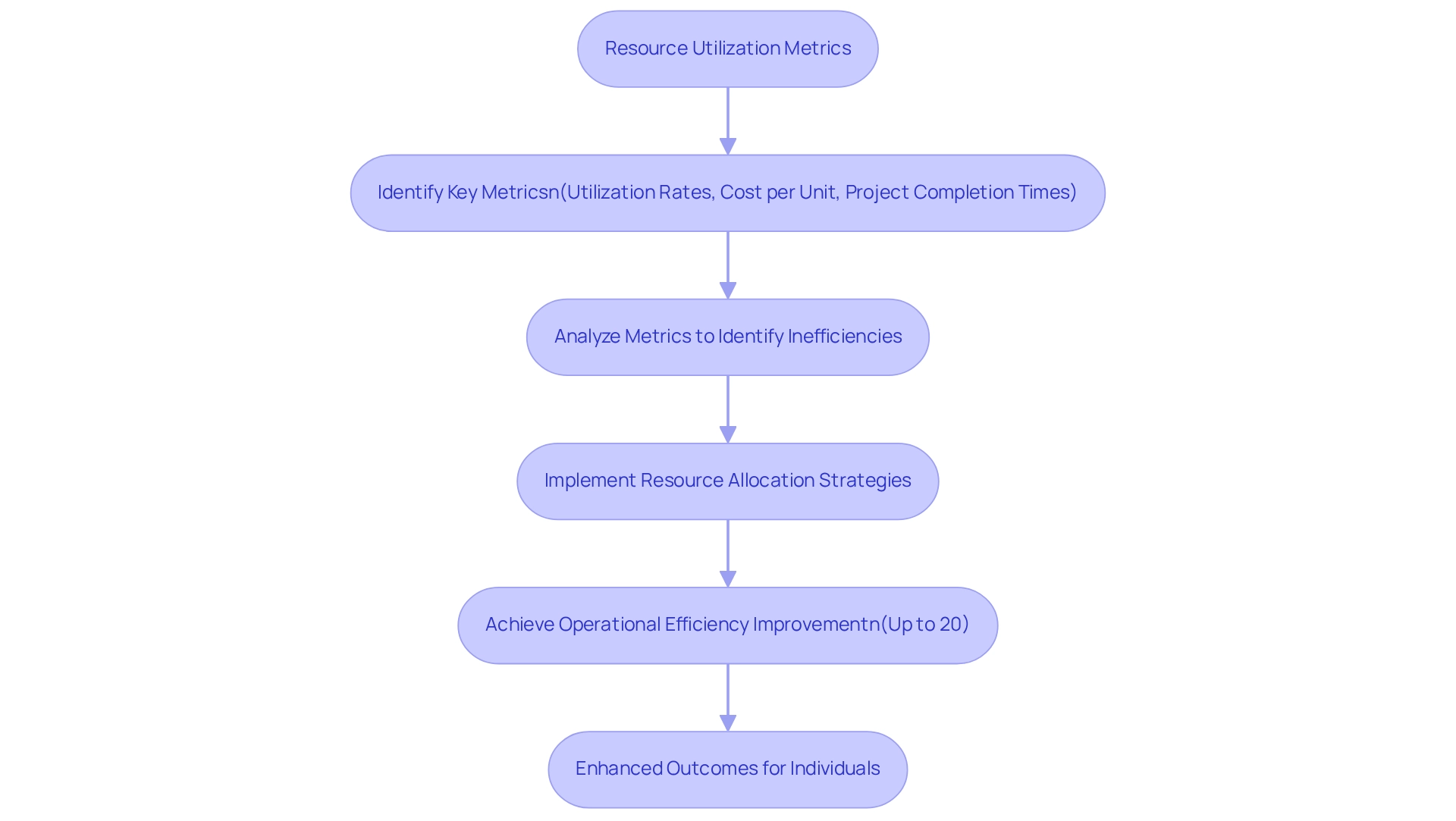
Resource Utilization Metrics: Optimizing Resource Allocation in Pharmaceuticals
Resource utilization metrics are a crucial process measure for drug manufacturers aiming to enhance their distribution of resources, including staff, equipment, and budget. Key metrics encompass process measures, including utilization rates, cost per unit produced, and project completion times. In 2025, the drug industry is witnessing a notable emphasis on these metrics, with companies leveraging advanced analytics to achieve up to a 20% improvement in operational efficiency through targeted resource allocation strategies.
By meticulously analyzing these metrics, which serve as a process measure, organizations can identify inefficiencies, streamline operations, and enhance overall productivity. Effective resource allocation is a vital process measure that not only leads to improved financial performance but also significantly enhances outcomes for individuals. Companies that implement a process measure in their data-driven approach to resource utilization are better positioned to navigate the complexities of the healthcare landscape.
Case studies illustrate the effective implementation of process measures related to resource utilization metrics in drug industry operations. CareSet’s Medicare data analysis, integrating over 100 external data sources, has empowered clients to make informed decisions that enhance patient care and improve strategic initiatives. This comprehensive view of the healthcare landscape acts as a process measure, enabling stakeholders to identify areas for improvement and optimize their resource allocation strategies effectively, particularly in the context of oncology treatment options.
As W. Edwards Deming observed, “The goal of leadership should be to enhance the performance of humans and machines, to elevate quality, to boost output…” This principle underscores the significance of leadership in optimizing resource distribution within drug manufacturing operations. As the industry evolves, the focus on optimizing resource allocation will continue to intensify, with process measures becoming increasingly sophisticated. In 2025, drug companies are anticipated to embrace innovative practices that not only enhance operational efficiency but also ensure that resources are utilized effectively to meet the demands of a dynamic market. Ultimately, the integration of robust resource utilization metrics as a process measure, supported by CareSet’s insights, will be essential for driving success in the pharmaceutical sector.
Conclusion
Data-driven methodologies are fundamentally transforming the pharmaceutical landscape, equipping companies with essential tools to enhance patient care and optimize operations. Through comprehensive Medicare data analysis by CareSet, alongside the implementation of the Balanced Scorecard and Statistical Process Control, organizations can pinpoint inefficiencies, monitor compliance, and elevate overall performance. The insights derived from these methodologies extend beyond theoretical frameworks; they manifest in real-world applications that can lead to improved health outcomes and heightened operational efficiency.
As the pharmaceutical industry evolves, integrating advanced analytics and key performance indicators becomes paramount for companies seeking to navigate complexities and foster long-term strategic growth. Patient journey mapping and engagement metrics exemplify how a deep understanding of patient interactions and preferences can significantly enhance treatment adherence and overall satisfaction. Furthermore, a focus on compliance metrics ensures that organizations not only meet regulatory standards but also cultivate trust and credibility with stakeholders.
Ultimately, embracing these innovative approaches is crucial for pharmaceutical companies striving to remain competitive in a rapidly changing market. By leveraging data-driven insights, organizations can refine their strategies, deliver value to patients, and achieve sustainable success in the healthcare sector. The future of pharmaceuticals hinges on the capacity to harness these insights, driving meaningful improvements that benefit both patients and the industry as a whole.


