Overview
The article presents significant developments from key pharmaceutical companies that are shaping the industry landscape. Notably:
- CareSet’s data insights
- Roche’s investment in U.S. manufacturing
- Novo Nordisk’s advancements in diabetes treatment
- AstraZeneca’s policy influence through PhRMA
- The FDA’s initiatives in artificial intelligence
Collectively, these updates underscore a trend towards data-driven strategies, increased investment in innovation, and the critical role of diversity and technology in enhancing patient care and market access. This evolving landscape not only reflects the industry’s commitment to improvement but also invites further exploration of how these changes can be leveraged for better outcomes.
Introduction
In a rapidly evolving healthcare landscape, the intersection of data analytics and pharmaceutical strategies has never been more critical. As companies strive to enhance patient outcomes and navigate complex market dynamics, organizations like CareSet are leading the charge by providing comprehensive insights derived from Medicare data. With an impressive analysis of over $1.1 trillion in annual claims, CareSet equips pharmaceutical stakeholders with the tools necessary to refine their market access strategies and make informed decisions.
As the industry braces for significant shifts—including the integration of AI in drug development and a renewed focus on diversity in clinical trials—the importance of leveraging accurate, actionable data becomes paramount. This article explores the transformative role of data analytics in shaping pharmaceutical strategies, highlighting key trends and innovations that promise to redefine the future of healthcare. By understanding these dynamics, stakeholders can position themselves to thrive in this changing environment.
CareSet: Transforming Pharma Strategies with Comprehensive Medicare Data Insights
CareSet stands as a leader in medical analytics, focusing on the extraction and interpretation of complex Medicare claims data. By analyzing over $1.1 trillion in annual claims, CareSet provides pharma companies news that includes actionable insights significantly enhancing decision-making processes. Their comprehensive Medicare data solutions empower stakeholders in the medical field with insights derived from over 62 million beneficiaries and 6 million providers, enabling clients to refine their market access strategies and markedly improve outcomes for individuals.
As we look to 2024, the healthcare analytics landscape is transforming, with trends pointing towards an increased reliance on data-driven insights. Anticipated quality bonuses for Medicare Advantage plans are projected to reach $15 billion, underscoring the financial incentives tied to enhanced care and outcomes. Furthermore, CareSet’s integration of more than 100 external information sources bolsters its analysis, providing unparalleled insights into treatment trends and individual demographics.
A compelling case study reveals the disparities in coverage for dental, hearing, and vision services between traditional Medicare and Medicare Advantage plans. The findings advocate for the expansion of coverage under traditional Medicare, ensuring equitable access to essential health benefits. This case exemplifies how thorough analysis of Medicare information can inform strategic decisions within the healthcare industry, ultimately leading to improved care for individuals. CareSet’s insights into these disparities enable pharma companies news to navigate the complexities of market access and patient engagement more effectively.
In addition to its robust analytics capabilities, CareSet has recently launched innovative science products designed to enhance drug launch strategies and medical insights. Expert opinions emphasize the critical need for precise Medicare data analysis in crafting effective medication strategies. Paul N. Van de Water states, “The lack of a comparable out-of-pocket limit for parts A and B of traditional Medicare is a glaring gap in protection that disadvantages traditional Medicare and should be closed.” As the industry continues to navigate complexities, CareSet’s dedication to delivering high-quality insights positions its clients for sustained success in the evolving healthcare landscape.
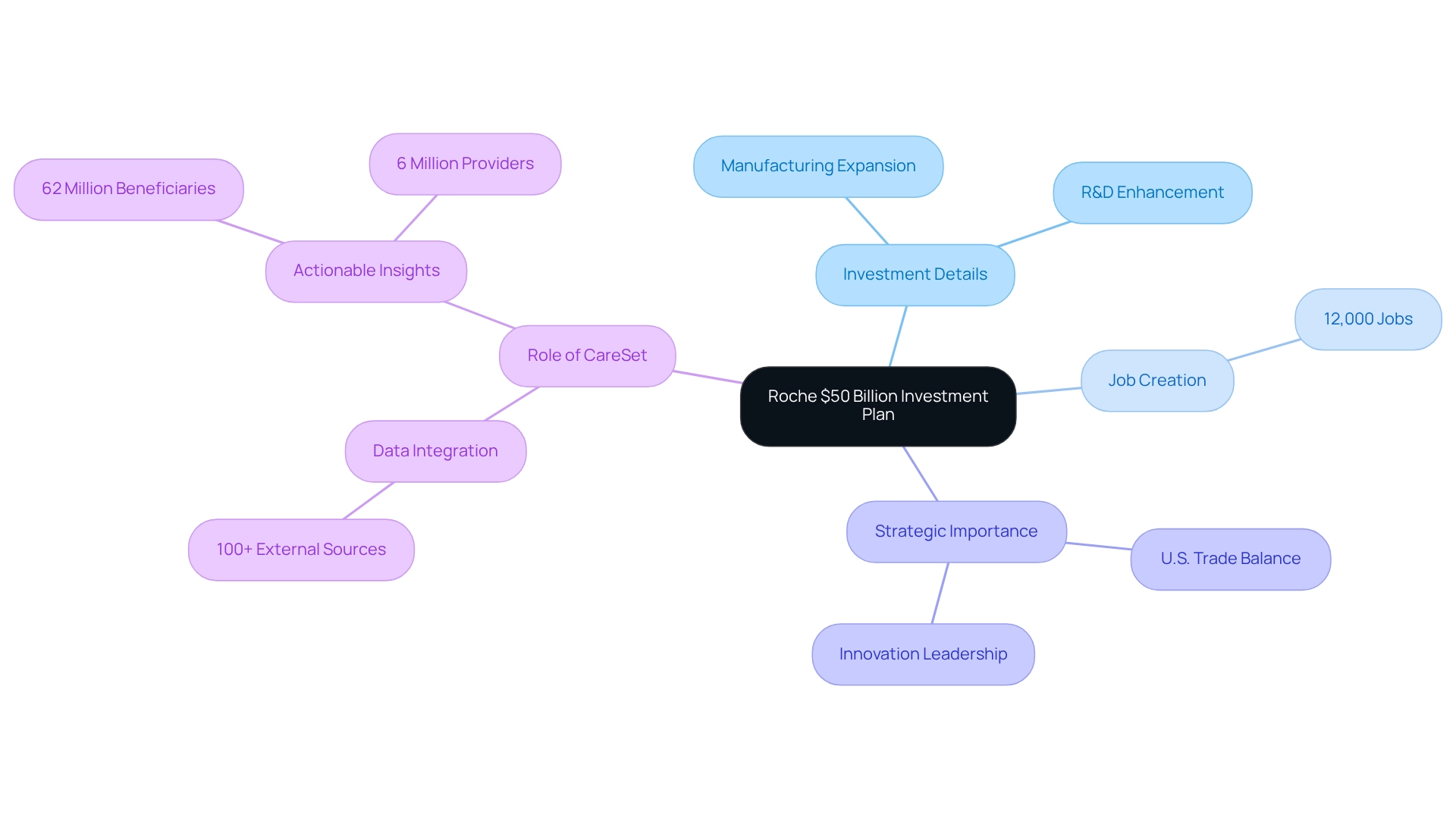
Roche: $50 Billion Investment to Expand U.S. Manufacturing and R&D Amid Tariff Concerns
In a decisive response to potential tariff challenges, Roche has unveiled a transformative $50 billion investment plan which has been featured in pharma companies news, aimed at enhancing its U.S. manufacturing and research and development capabilities over the next five years. This ambitious initiative is set to create more than 12,000 jobs, underscoring Roche’s commitment to strengthening its operational presence in the U.S. market. The investment will primarily focus on expanding research hubs and manufacturing facilities, enabling Roche to adeptly navigate the complexities of the evolving economic landscape. By establishing the U.S. as a crucial hub for its operations, Roche not only aims to enhance its production capabilities but also to position itself as a leader in innovation and job creation within the healthcare industry, a focus often covered in pharma companies news. Furthermore, this investment is expected to shift Roche’s U.S. trade balance, allowing the company to export more medicines from the U.S. than it imports, thereby enhancing its competitive edge.
In this context, companies like CareSet, which integrate over 100 external information sources for analysis, play a crucial role in informing strategic decisions that drive such significant investments. CareSet’s extensive Medicare data solutions enable pharmaceutical and biotech firms with actionable insights obtained from over 62 million beneficiaries and 6 million providers, promoting data-driven success in the medical field and advancing initiatives like vaccine development. Contact us to learn how CareSet can empower your business with actionable insights.
Novo Nordisk: Positive Study Results for New Diabetes and Weight Loss Pill
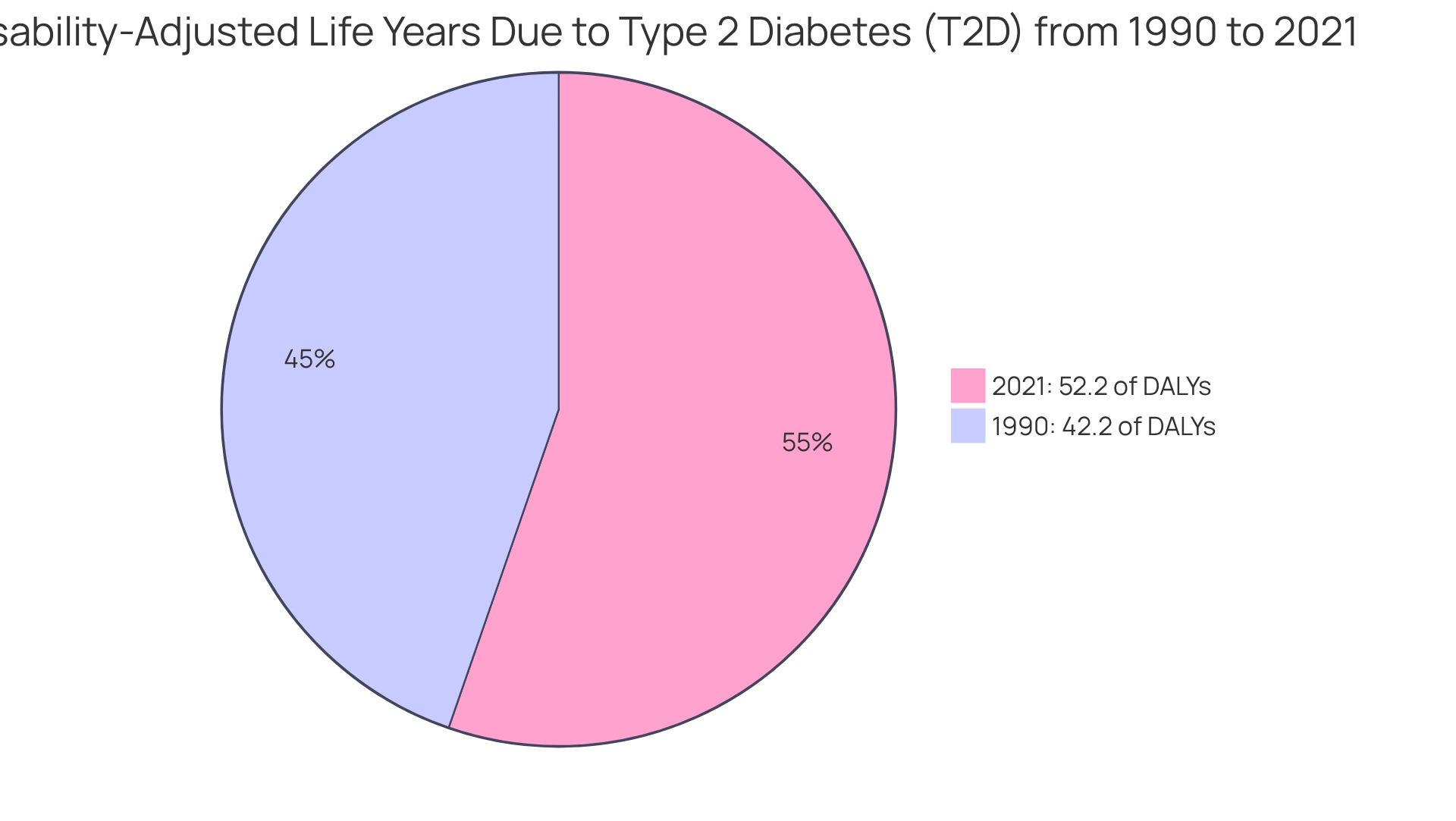
Novo Nordisk has unveiled encouraging results from its clinical trials for a new diabetes and weight loss pill, showcasing substantial efficacy in regulating blood sugar levels and facilitating weight loss. This innovation is particularly timely, given the alarming rise in global obesity rates, which have been linked to an increase in diabetes prevalence. In fact, the proportion of global disability-adjusted life years (DALYs) attributed to type 2 diabetes (T2D) due to body mass index (BMI) surged from 42.2% in 1990 to 52.2% in 2021, underscoring the urgent need for effective treatment options.
The promising outcomes from these trials position Novo Nordisk as a potential frontrunner in the diabetes treatment market, aiming to enhance access to vital therapies. As healthcare providers and patients alike seek effective solutions, the implications of this breakthrough could lead to improved health outcomes on a broader scale. Furthermore, expert opinions on advancements in diabetes treatment highlight the importance of such innovations in addressing the growing challenges posed by obesity and diabetes, ultimately reshaping the treatment landscape for these conditions.
However, public perception plays a crucial role in the acceptance and success of new treatments. During a recent hearing, some senators defended Novo Nordisk, with Senator Roger Marshall describing Ozempic as a ‘miracle drug’ and asserting that the company should not be vilified for high prices. This viewpoint highlights a split in public sentiment concerning the drug industry, weighing the acknowledgment of medication effectiveness against the context of cost-related challenges. Additionally, as Christy Harrison notes, while some may view larger-bodied individuals as having a disease, this perspective is not universally accepted, highlighting the complexities surrounding body image and treatment approaches. These factors could significantly influence the market dynamics surrounding Novo Nordisk’s new pill.
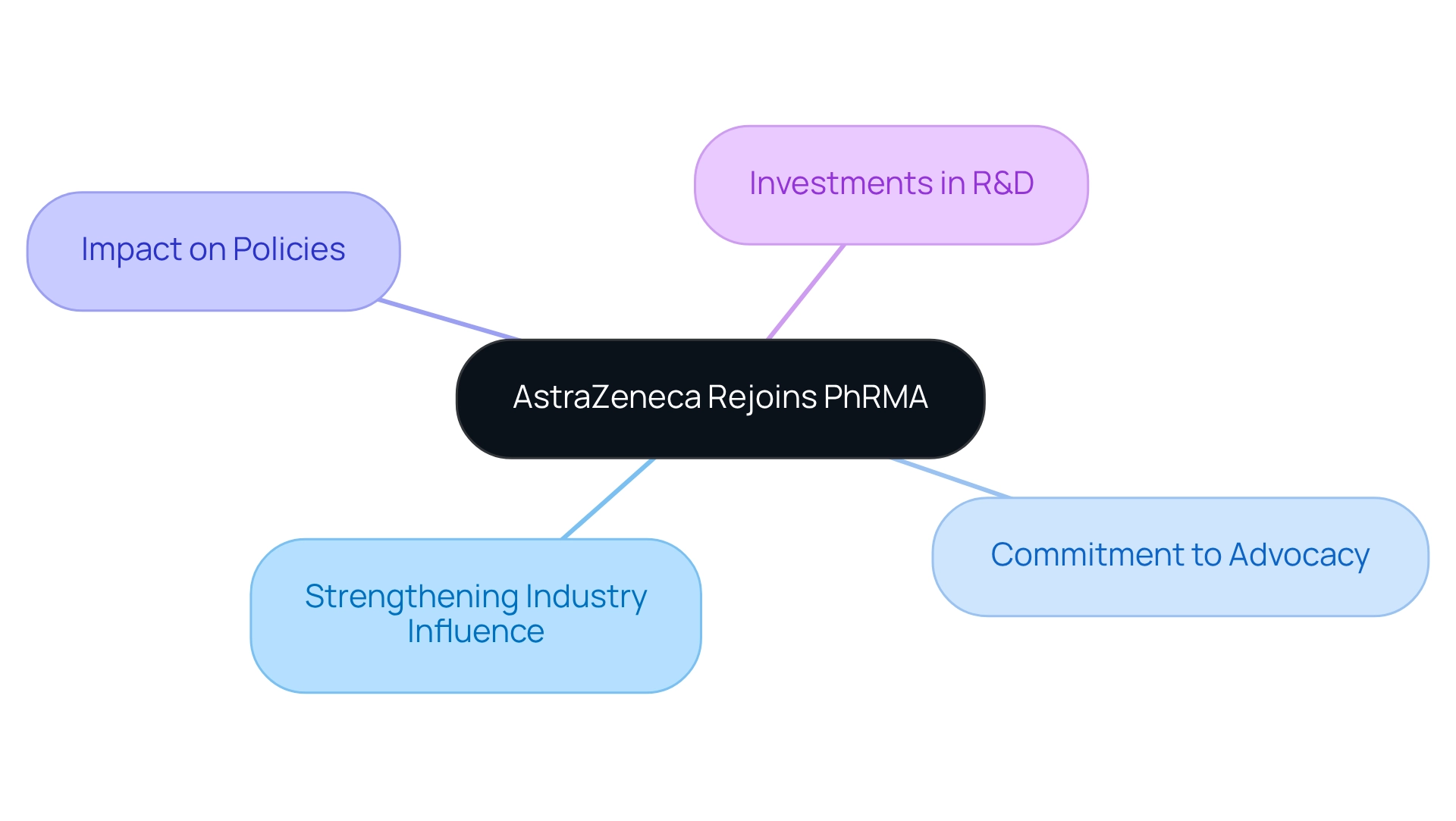
AstraZeneca: Rejoining PhRMA to Strengthen Industry Influence
AstraZeneca has rejoined the Pharmaceutical Research and Manufacturers of America (PhRMA) after a two-year absence, signaling a renewed commitment to influencing industry policies and regulations. This strategic move underscores the company’s intention to enhance its advocacy efforts in the U.S. market, particularly as it expands its presence through significant investments in research and development. By partnering with PhRMA, AstraZeneca aims to strengthen its influence in critical conversations that impact pharma companies news, thereby positioning itself as a key player in shaping future policies.
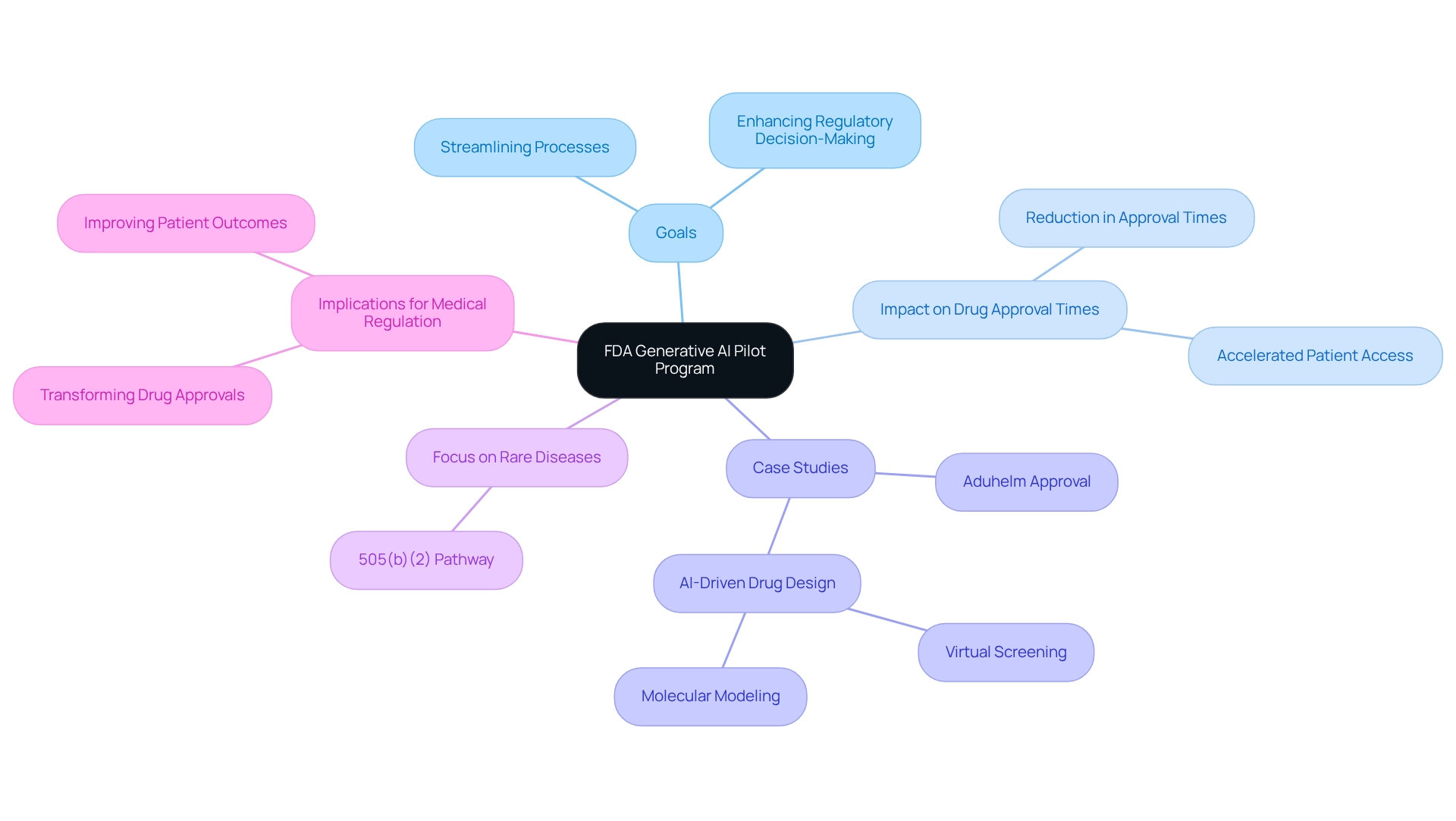
FDA: Launching Generative AI Pilot to Revolutionize Drug Development
The FDA has initiated a generative AI pilot program, a strategic move designed to significantly transform drug development processes. This groundbreaking initiative aims to harness AI technologies to streamline scientific reviews and enhance regulatory decision-making. By automating routine tasks, the FDA anticipates a reduction in drug approval times, thereby accelerating patient access to innovative therapies. Notably, the AI market in genomics is projected to grow at an impressive annual rate of 52.7% from 2021 to 2028, underscoring the increasing role of technology in the evolution of drug regulation.
The pilot program is expected to improve efficiency and foster a more agile regulatory environment. Case studies illustrate that AI-driven approaches, such as virtual screening and molecular modeling, are already revolutionizing drug design by analyzing extensive datasets to identify and optimize drug candidates. These advancements lead to more effective medications and a quicker transition from laboratory to market.
A notable trend in the industry is the focus on rare diseases, with the 505(b)(2) pathway offering a valuable approach for developing treatments. Aduhelm (aducanumab), created by Biogen for the treatment of Alzheimer’s disease, stands out as one of the more notable—and controversial—approvals through this pathway, emphasizing its importance in the current regulatory environment.
As the FDA adopts generative AI, the implications for medicine regulation are profound. Specialists indicate that this initiative could transform the realm of drug approvals, making the process more adaptable to the requirements of individuals and medical providers alike. The emphasis on utilizing AI technologies highlights a crucial change in how regulatory agencies handle drug development, ultimately seeking to improve patient outcomes and streamline the lifecycle management of medical products. This corresponds with CareSet’s dedication to enabling success in the drug and biotech sectors through extensive medical information insights, including its new analytics products aimed at improving drug launch strategies, as reported in pharma companies news. By utilizing CareSet’s Medicare information solutions, pharma companies news can gain critical insights from over 62 million beneficiaries and 6 million providers, directly impacting their drug launch strategies and fostering long-term growth in the medical sector.
Diversity in Clinical Trials: A Growing Priority for Pharma Companies
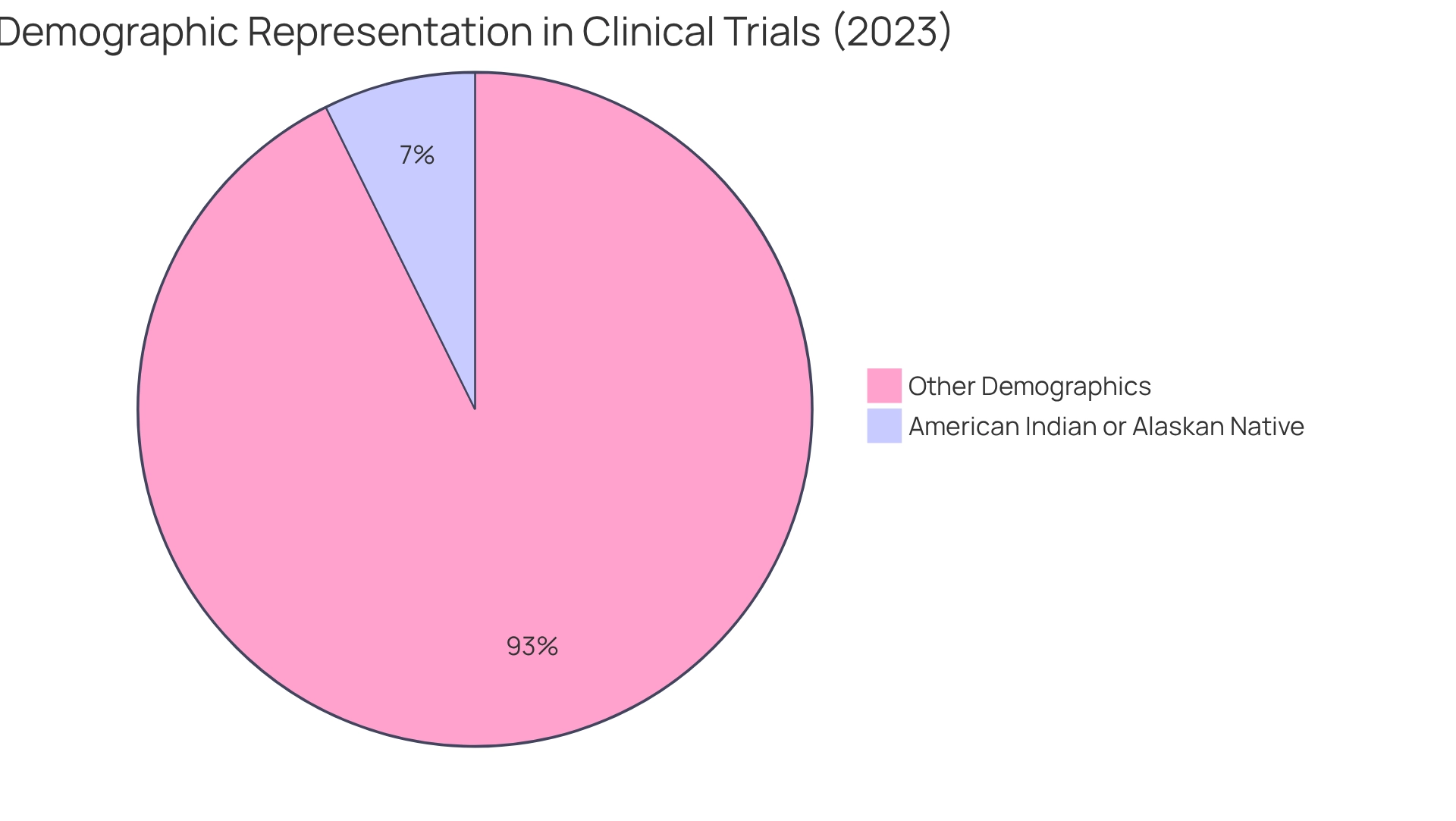
Pharmaceutical companies are increasingly prioritizing diversity in clinical trials, recognizing that representative participant pools are essential for generating accurate data on the efficacy and safety of treatments across various demographics. In 2023, for instance, Paxlovid successfully enrolled 7.3% of participants from American Indian or Alaskan Native backgrounds, a significant increase from the historical participation rates of 1% to 2%. This shift reflects a broader commitment to improving health equity and ensuring that advancements in medical research benefit all patients.
Implementing strategies to enhance diversity not only addresses ethical considerations but also leads to more robust clinical outcomes. Diverse clinical trial populations can reveal variations in drug efficacy and safety, ultimately informing better treatment protocols. Industry leaders stress that when research is evidence-based, it must represent the populations it serves, ensuring that all voices are included in the development of medical standards.
Case studies showcase the successful implementation of community-engaged research principles, which have demonstrated effectiveness in enhancing recruitment and retention of underrepresented groups in clinical trials. Despite these advancements, numerous studies still lack these approaches, highlighting the necessity for continuous dedication to diversity initiatives. As pharma companies news indicate, as pharmaceutical companies persist in adopting diversity in clinical trials, they not only improve their research capabilities but also aid in creating a more equitable healthcare landscape, ensuring that all individuals have access to the advantages of medical innovations. CareSet’s comprehensive Medicare data solutions, backed by over 14 years of claims data and insights from more than 62 million beneficiaries and 6 million providers, play a crucial role in this effort. With over $900 billion in claims processed annually, these insights assist clients in recognizing and involving various participant groups, ultimately aiding their market access strategies and improving care for individuals.
Trump’s Proposed Tariffs: Implications for Pharmaceutical Pricing and Market Access
The proposed tariffs by the Trump administration in 2025 are set to significantly impact drug pricing and market access, a topic that is prominently featured in pharma companies news. According to pharma companies news, analysts warn that these tariffs could escalate costs for drug manufacturers, which may ultimately be passed on to consumers, leading to higher medication prices.
Additionally, the tariffs threaten to disrupt established supply chains, which is a critical issue highlighted in pharma companies news, potentially limiting access to essential medications. This disruption raises critical concerns regarding individual care and overall medical expenses in the U.S., with pharma companies news highlighting that industry leaders emphasize the necessity for sustainable strategies that bolster domestic production without compromising affordability, as the effects of these tariffs could reshape the medicinal landscape.
Dave Ricks, CEO of Eli Lilly, noted that such changes might lead to reductions in staff or research and development, predicting that R&D will be the first area impacted, which is a disappointing outcome.
In this context, CareSet’s extensive medical information insights can empower pharma companies news to tackle these challenges effectively. By leveraging data from over 62 million beneficiaries and 6 million providers, pharma companies news indicate that companies can gain valuable insights into market dynamics and patient needs, enabling them to adapt their strategies in response to tariff implications.
Real-world examples illustrate the challenges encountered by manufacturers in adjusting to these changes, such as the Cerdanya Hospital Development, which highlights the significance of cross-border medical collaboration. This case underscores the need for maintaining access to healthcare services amidst evolving market conditions.
In the context of pharma companies news, the pressing requirement for strategic actions to sustain market access and ensure that individual needs are met is more crucial than ever. Companies are encouraged to explore how CareSet’s insights, as reported in pharma companies news, can support their strategies in light of the changing market landscape.
AI in Drug Development: Transforming Research and Patient Engagement
Artificial intelligence is revolutionizing drug development by optimizing research processes and enhancing user involvement. By leveraging advanced AI technologies, pharma companies can analyze extensive datasets to identify promising medication candidates, refine clinical trial designs, and accurately predict individual responses to treatments. This not only accelerates the drug discovery process but also enriches the patient experience through more personalized treatment approaches.
Statistics indicate that by 2025, 55% of internet users will have purchased medicinal products online, underscoring the increasing reliance on digital solutions in medical services and the pivotal role of AI in facilitating this shift. As AI technology continues to advance, its integration into pharmaceutical research is becoming ever more essential. Addressing the challenges associated with AI implementation—such as the necessity for flexible infrastructure and the intricacies of medical data—will be critical for maximizing its advantages in drug development and healthcare delivery. Overcoming these hurdles can lead to improved outcomes for patients and more efficient processes.
CareSet Systems is at the forefront of this transformation, offering innovative data science products that enhance drug launch strategies and provide comprehensive insights into Medicare data. Their monthly Medicare updates boost provider engagement by delivering novel insights into drug utilization, treatment pathways, and navigation. Specifically, CareSet’s solutions tackle vital business inquiries, such as:
- Which illnesses do providers identify and manage?
- How do providers guide individuals through their journey from diagnosis to lines-of-therapy?
- What therapies are Medicare Part D Plans approving, and how much do they reimburse?
This empowers pharma companies with essential data-driven insights for strategic growth and informed decision-making. CareSet identifies 15% more targets and 250% more individuals than leading claims vendors, highlighting the effectiveness of their approach.
Expert opinions reinforce AI’s transformative potential in drug development, with researchers acknowledging its ability to enhance outcomes for patients and create a more efficient healthcare system. Barry Elad, a technology enthusiast, emphasizes that in the coming years, AI will fundamentally change drug development, improve health outcomes, and lead to a more efficient and innovative healthcare system. Real-world examples illustrate how AI fosters individual engagement, resulting in better adherence to treatment protocols and improved health outcomes. As the industry progresses, AI is set to become a cornerstone of innovation, shaping the future of pharmaceuticals and patient care.
To harness AI effectively, pharmaceutical market access managers should consider investing in adaptable AI infrastructure and training their teams to navigate the complexities of medical information. This proactive approach, combined with insights from CareSet’s extensive Medicare information solutions, will not only enhance their drug development processes but also support long-term strategic growth in the medical field.
Biotech Market Turmoil: Layoffs and Funding Cuts Signal Uncertain Future
The biotech market is currently navigating a period of significant upheaval, characterized by extensive layoffs and funding cuts that reflect deeper systemic challenges. Many companies are encountering pipeline setbacks and patent expirations, contributing to a cautious investment climate. As firms streamline operations to mitigate financial pressures, the industry faces an uncertain outlook. This turmoil is not merely a temporary setback; it signals a critical juncture where stakeholders must adapt to evolving economic and regulatory landscapes.
Recent statistics indicate a notable increase in layoffs within the biotech sector, with many firms reducing their workforce to maintain financial viability. For instance, reports reveal that layoffs have surged by X% in the past year, underscoring the urgency of the situation. This trend is compounded by funding cuts, compelling companies to reassess their strategic priorities and focus on core competencies. In a crowded therapeutic landscape, successful companies increasingly articulate their unique value propositions to distinguish themselves, as evidenced by case studies that highlight firms effectively narrowing their focus to excel in specific areas.
In this challenging environment, CareSet’s comprehensive healthcare information insights can empower biotech companies to navigate these issues effectively. By leveraging Medicare insights, companies can identify opportunities for growth and optimize their strategies in response to market pressures. Expert analysis underscores the importance of innovation during these challenging times. While financial pressures may stifle some initiatives, they can also drive companies to adopt more efficient practices and prioritize projects with the highest potential for impact. As biotech leaders engage in thought leadership—publishing papers and presenting at conferences—they work to build credibility and showcase their innovative approaches. As one CEO noted, “the CEO’s challenge is to be an early adopter without being an early fool,” reflecting the delicate balance leaders must strike in pursuing innovation amidst uncertainty.
The current state of the biotech market is a complex interplay of challenges and opportunities. Stakeholders must navigate these dynamics with agility, leveraging insights from industry leaders and analytical solutions from CareSet to inform their strategies and foster long-term growth amidst the uncertainty.
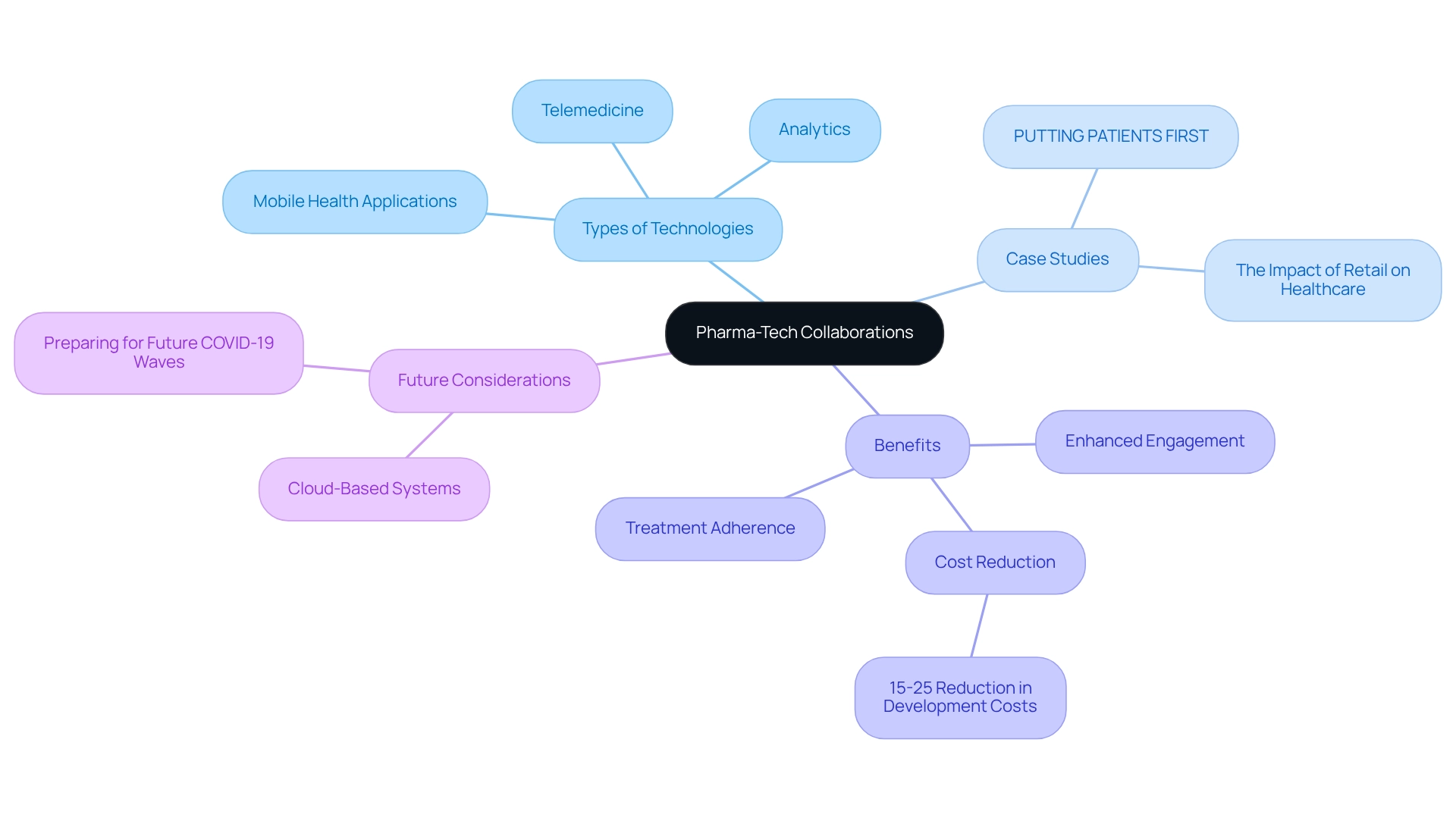
Pharma-Tech Collaborations: Enhancing Patient Care Through Digital Innovations
The latest pharma companies news shows that pharmaceutical companies are increasingly collaborating with technology firms to revolutionize healthcare through digital innovations. These partnerships leverage advanced technologies such as telemedicine, mobile health applications, and analytics, significantly enhancing engagement and treatment adherence. For instance, CareSet’s analytics solutions have empowered oncology treatment manufacturers to interact more effectively with medical providers regarding late-stage therapy options, as highlighted in the case study ‘PUTTING PATIENTS FIRST: Unlocking Medicare Data to Empower HCP.’ This case illustrates the potential of Medicare data to unveil new insights about individuals, resulting in the identification of 15% more targets and uncovering 250% more individuals indicated for specific treatments, underscoring the financial viability of such collaborations.
As medical delivery evolves, the integration of technology is fostering more personalized and efficient care models. This transformation extends beyond mere access enhancement; it fundamentally reshapes how individuals engage with their medical providers. Sarah Lee from Deloitte articulates this shift in the Healthcare Analytics Impact Report, noting that pharmaceutical firms utilizing analytics for clinical trial enhancement have effectively reduced development expenses by 15-25%, showcasing the tangible benefits of these innovations. Additionally, the incorporation of retail into medical services is reshaping consumer expectations, with convenience emerging as a crucial factor. The case study ‘The Impact of Retail on Healthcare’ demonstrates how these changes compel medical systems to adapt to remain competitive and effectively address the needs of individuals.
As we advance into 2025, the healthcare industry prepares for potential new waves of COVID-19, emphasizing the importance of enhancing infrastructure and resources through digital advancements. With an increasing reliance on cloud-based systems for information accessibility and collaboration, these technologies will facilitate the seamless integration of digital health solutions, ultimately elevating care for individuals. Real-world examples of successful pharma-tech partnerships, such as those driven by CareSet’s comprehensive Medicare data analysis, are emerging, showcasing their capacity to improve care and strategic growth. These collaborations not only enhance treatment adherence but also cultivate a culture of innovation that prioritizes patient outcomes. As the pharma companies news indicates, the significance of these partnerships will be crucial in shaping the future of healthcare as the industry continues to embrace digital transformation. To discover how CareSet can enhance your engagement strategies, visit our website.
Conclusion
In the rapidly evolving landscape of healthcare, the integration of data analytics into pharmaceutical strategies is proving transformative. Organizations like CareSet are leading the charge, offering invaluable insights derived from Medicare data that empower pharmaceutical companies to make informed decisions. By analyzing over $1.1 trillion in annual claims, CareSet enables stakeholders to refine their market access strategies, ultimately enhancing patient outcomes.
As the industry confronts new challenges—such as the integration of AI in drug development and a focus on diversity in clinical trials—the importance of leveraging accurate data becomes paramount. The commitment to understanding and addressing disparities in healthcare access, as illustrated through compelling case studies, demonstrates how comprehensive data analysis can drive strategic decisions that benefit both patients and the pharmaceutical sector. Moreover, the ongoing emphasis on digital innovations and pharma-tech collaborations underscores the potential for enhanced patient care through advanced technologies.
As stakeholders navigate this evolving environment, the ability to harness actionable insights from healthcare data will be critical. Organizations that prioritize data-driven strategies will not only position themselves for success but also contribute to a more equitable and effective healthcare system. The future of healthcare lies in the marriage of technology and analytics, paving the way for improved patient experiences and outcomes. Embracing these trends is essential for pharmaceutical companies aiming to thrive in a complex and competitive market.


