Overview
The article titled “10 Key Trends in Pharma Industry News You Need to Know” centers on significant developments and shifts within the pharmaceutical industry that stakeholders must recognize. While the specific content of the article is not provided, it indicates that these trends cover essential areas such as:
- Regulatory changes
- Technological advancements
- Investment strategies
- Market dynamics
Understanding and adapting to these trends is crucial for companies operating in the pharma sector to ensure future success. By staying informed on these key developments, stakeholders can position themselves strategically in a rapidly evolving landscape.
Introduction
In the rapidly evolving landscape of the pharmaceutical industry, data-driven insights are becoming indispensable for strategic decision-making. Companies are increasingly turning to advanced analytics to navigate complex challenges, from regulatory changes to market access strategies. CareSet, a leader in healthcare analytics, exemplifies this trend by leveraging vast amounts of Medicare claims data to uncover critical insights that inform treatment patterns and patient engagement.
As healthcare spending continues to rise, the integration of innovative technologies and comprehensive data solutions is essential for pharmaceutical companies aiming to enhance patient care and optimize their operations. This article delves into how various industry players, including:
- Roche
- Johnson & Johnson
- Regeneron
are utilizing these insights to drive growth and improve health outcomes amidst a backdrop of regulatory shifts and market uncertainties.
CareSet: Leveraging Medicare Data for Strategic Insights
CareSet stands at the forefront of medical analytics, focusing on the extraction and interpretation of intricate Medicare claims information. With an analysis of over $1.1 trillion in annual claims, CareSet delivers essential insights into treatment patterns, provider networks, and demographics. This comprehensive, insights-driven approach empowers drug companies and medical organizations to identify new provider targets, examine prescribing habits, and chart individual treatment paths, thereby enhancing care and guiding strategic initiatives.
By 2025, the significance of Medicare information in the drug sector is underscored by the increasing share of medical spending, projected to rise from 17.6% of GDP in 2020 to 19.7% by 2032. This trend in pharma industry news highlights the necessity for pharmaceutical companies to harness Medicare insights for effective market access and consumer engagement strategies. Experts in medical analytics assert that the focus should extend beyond merely adapting to changes; it should also encompass leveraging insights to enhance service delivery and equity in patient care, as noted by Sara Vaezy.
Current trends indicate that medical systems must innovate based on insights gleaned from the pandemic, rendering the analysis of Medicare claims information more crucial than ever, particularly in light of escalating medical expenditures. CareSet’s integration of over 100 external information sources enriches its insights, enabling clients to adeptly navigate the complexities of the healthcare landscape. Successful case studies, including one centered on oncology treatment options like Qinlock for Gastrointestinal Stromal Tumor (GIST), demonstrate how Medicare analysis has led to improved treatment analytics and expanded market reach, which is significant in the context of pharma industry news. Furthermore, CareSet’s innovative analytics solutions, featuring advanced analysis tools and real-time information integration capabilities, are designed to enhance drug launch strategies, reaffirming the importance of evidence-based methods in optimizing product lifecycle management. Additionally, CareSet addresses common inquiries regarding its services through comprehensive FAQs, ensuring clients are well-informed and equipped to effectively utilize Medicare data.
Roche: $50 Billion Investment in U.S. Manufacturing and R&D
Roche has unveiled a groundbreaking $50 billion investment in U.S. manufacturing and research and development over the next five years. This ambitious initiative is projected to generate over 12,000 jobs, significantly enhancing Roche’s footprint in the U.S. market. The investment will aid in the creation of new R&D centers and manufacturing facilities, strategically positioning Roche to reduce potential tariff effects and enhance its operational capabilities in a quickly changing healthcare environment.
Industry analysts regard this action as a crucial measure that not only strengthens Roche’s dedication to innovation but also aids in the overall expansion of the U.S. drug industry, which is frequently covered in pharma industry news. The expansion is expected to stimulate local economies and create a ripple effect in job opportunities across various sectors, further solidifying Roche’s role as a leader in the industry.
Additionally, CareSet’s expertise in integrating over 100 external data sources for comprehensive insights can provide valuable context for understanding the implications of such investments.
With access to over $1.1 trillion in yearly Medicare claims and 14 years of analytics, CareSet enables drug and biotech firms to utilize actionable insights for strategic growth. Their case study on long-term strategic growth for partners illustrates how Roche’s investment might foster similar growth opportunities in the industry.
Moreover, pertinent statistics on drug investments in U.S. manufacturing for 2025 highlight the importance of Roche’s initiative, while insights from pharma industry news and industry analysts can further clarify the effect of this investment on the U.S. drug market.
Veeva: Upgrading Life Science Data for Precision Targeting
CareSet Systems is at the forefront of enhancing medical strategies through innovative information science products designed to support drug launches and improve healthcare insights. Recently, CareSet introduced four new dynamic data science products that leverage extensive Medicare data, empowering pharmaceutical and biotech companies to navigate the complexities of market access and client engagement effectively. These products provide actionable insights into drug utilization, treatment pathways, and user navigation, which are vital for optimizing resource allocation in a competitive landscape.
The monthly Medicare updates from CareSet further enrich provider engagement by delivering novel insights into critical business questions, such as the diseases providers diagnose and treat, and how individuals navigate their treatment journeys. This information is indispensable for drug companies aiming to refine their strategies and improve health outcomes.
The integration of advanced analytics and AI-driven insights is poised to revolutionize the industry, with the AI sector in drug innovation projected to achieve a compound annual growth rate (CAGR) of 40.8% by 2024. This growth highlights the necessity of utilizing AI to enhance targeting precision, as recent findings indicate that AI has successfully associated new drugs approximately 25% of the time, a remarkable increase from the current market rate of 0.01%. As Barry Elad, a Senior Writer, notes, “Artificial Intelligence has been successful in associating new drugs almost 25% of the time, compared to the present market rate of 0.01%.”
Moreover, effective digital channels such as social media, email campaigns, and webinars are being harnessed to amplify outreach and engagement with medical providers and individuals. These channels not only foster community building but also facilitate targeted messaging, which is essential for maximizing marketing impact. For instance, drug marketing employs these digital platforms to craft customized campaigns that resonate with specific audiences, thereby enhancing overall engagement.
As CareSet continues to advance in healthcare analytics, the benefits of these innovations will be crucial for pharmaceutical firms that follow pharma industry news to refine their strategies and enhance outcomes in 2025 and beyond. Furthermore, collaboration with regulators can streamline AI development processes in pharma, further bolstering the effectiveness of these strategies.

Regeneron: Utilizing Real-World Evidence in Drug Development
Regeneron is at the forefront of integrating real-world evidence (RWE) into its drug development processes. By leveraging information from diverse sources such as electronic health records and registries, the company gains crucial insights into treatment effectiveness in real-world settings. This comprehensive approach not only bolsters regulatory submissions but also enhances clinical practice, ultimately resulting in improved patient outcomes and more effective therapies.
The incorporation of RWE is particularly crucial in 2025, as North America is anticipated to dominate the real-world information market. This trend underscores the importance of utilizing RWE to maintain competitiveness in the evolving medical landscape. For instance, the application of extensive data analysis and artificial intelligence in RWE studies has revolutionized the analytical process, enabling researchers to identify patterns and correlations that inform medical decision-making. Such advancements facilitate the examination of broader and more diverse populations, thereby enhancing the accuracy and scalability of studies.
Simultaneously, CareSet’s recent case study on oncology treatment alternatives illustrates how access to Medicare information can empower medical providers. By focusing on the 4th line of therapy for Gastrointestinal Stromal Tumor (GIST) with the treatment option Qinlock, CareSet demonstrates the potential of comprehensive Medicare solutions to refine medical strategies. This evidence-based approach not only fosters timely interactions with physicians but also directs treatment pathways, ultimately improving patient outcomes.
CareSet’s commitment to leveraging Medicare information is evident in its efforts to provide insights that align with actual patient needs. By integrating these data solutions into pharmaceutical strategies, CareSet ensures that medical providers are equipped with the essential information to enhance treatment outcomes. This strategic focus on data-driven insights is critical for improving the overall efficiency of medical service delivery. Furthermore, a recent case analysis highlighted how the integration of electronic health records has significantly improved treatment results by offering real-time information that informs clinical decisions. This example underscores the transformative potential of RWE in enhancing care for individuals and emphasizes the importance of CareSet’s strategy within the current medical landscape.

Johnson & Johnson: Promoting Diversity in Clinical Trials
Johnson & Johnson stands at the forefront of promoting diversity in clinical trials, recognizing the necessity for research to accurately reflect the diverse populations that will ultimately utilize their products. By implementing targeted strategies to engage underrepresented groups, the company enhances the inclusivity of its clinical research initiatives. This commitment not only enriches the quality of data collected but also builds trust and fosters engagement among diverse patient populations, which is crucial for achieving better health outcomes.
In 2025, the importance of diversity in clinical research is underscored by statistics indicating that diverse populations can significantly influence clinical trial outcomes. Engaging underrepresented groups broadens the participant base and ensures that findings are applicable across various demographics. For instance, Johnson & Johnson’s initiatives have been pivotal in addressing disparities in healthcare access and treatment efficacy.
Expert opinions emphasize that inclusivity in clinical research is not merely a regulatory requirement but a moral imperative that can lead to more effective and personalized treatment options. Leaders at Johnson & Johnson have articulated strategies focused on outreach and education to engage these populations, reinforcing the notion that diverse clinical trials yield more comprehensive insights into treatment responses.
Case studies illustrate the positive impact of diversity initiatives in clinical trials, such as the recent acquisition of EmVenio Research by PCM Trials, which aims to enhance the capabilities of decentralized clinical trials and support the FDA’s diversity initiatives. This acquisition exemplifies how firms are actively striving to enhance inclusivity in clinical research.
As the drug industry continues to develop, the focus on diversity will remain an essential element in improving the relevance and effectiveness of clinical research.

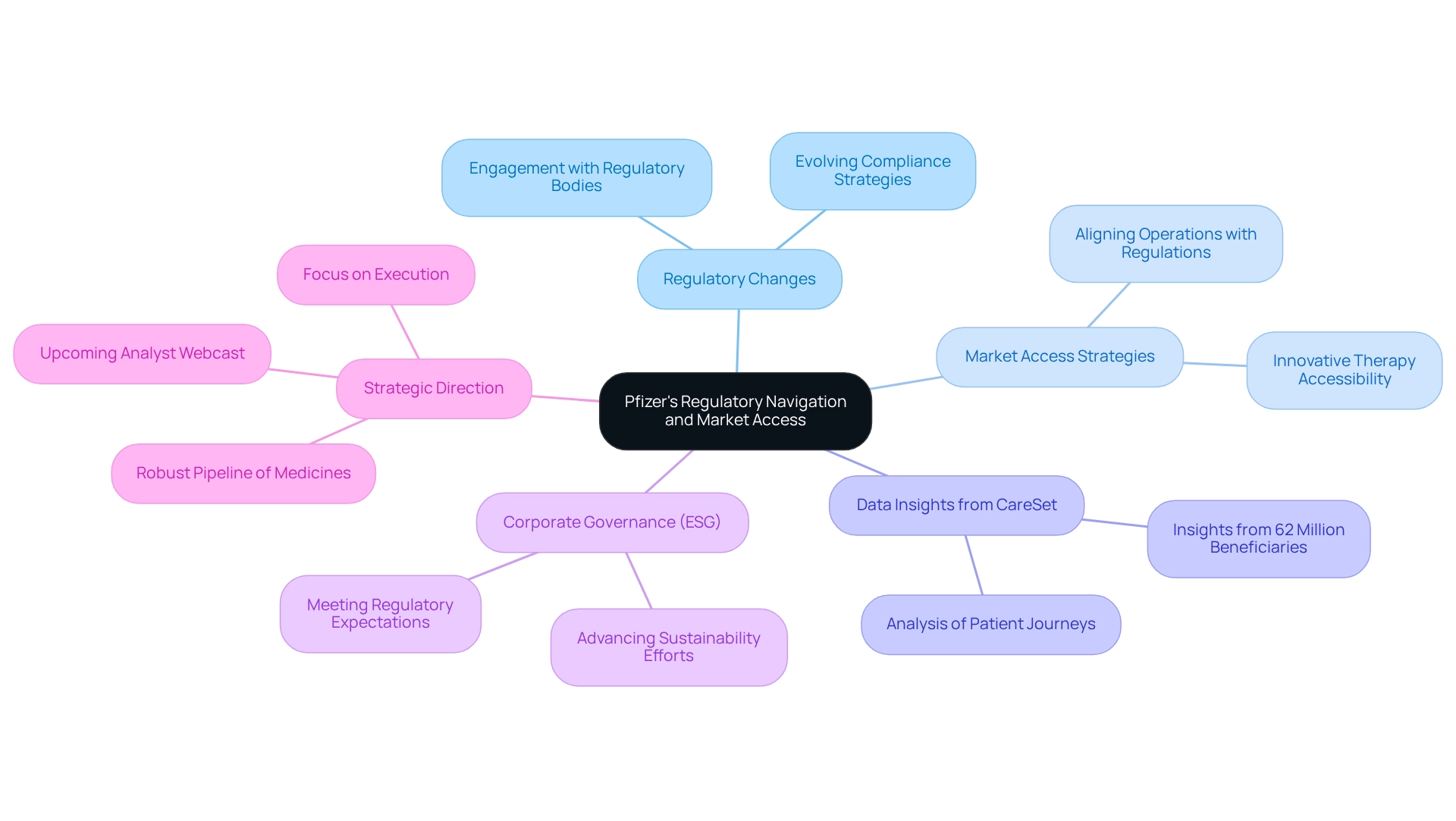
Pfizer: Navigating Regulatory Changes and Market Access
Pfizer is adeptly navigating an environment marked by evolving regulatory adjustments to sustain its market access and ensure that individuals can benefit from its innovative therapies. By actively engaging with regulatory bodies and refining its compliance strategies, Pfizer aims to mitigate risks associated with new policies. This proactive approach not only safeguards its product pipeline but also enhances its reputation as a leader in patient-centric care.
As the biologics market expands at an impressive compound annual growth rate of 15% projected until 2027, Pfizer’s commitment to adapting its market access strategies is vital. The firm is focusing on aligning its operations with recent regulatory modifications, which are increasingly influencing how medicines reach individuals. For example, Pfizer’s recent initiatives demonstrate a strategic response to the regulatory landscape, ensuring that its innovative therapies remain accessible.
In this context, leveraging comprehensive Medicare information solutions from CareSet can empower Pfizer and other pharmaceutical stakeholders with actionable insights. CareSet’s solutions analyze individual journeys and treatment pathways, providing essential insights into how individuals progress from diagnosis to treatment. By utilizing data from over 62 million beneficiaries and 6 million providers, Pfizer can enhance its understanding of market dynamics and refine its market access strategies.
Industry analysts have highlighted that navigating these regulatory challenges is critical for maintaining a competitive edge. Insights from experts underscore that effective market access strategies must evolve alongside regulatory changes, emphasizing the importance of compliance in ensuring access to therapies.
Furthermore, case studies illustrate Pfizer’s dedication to Environmental, Social, and Governance (ESG) priorities, which are increasingly intertwined with regulatory compliance. By advancing its sustainability efforts, Pfizer not only meets regulatory expectations but also enhances its corporate citizenship, further solidifying its market position.
In a recent statement, Dr. Albert Bourla, Chairman and Chief Executive Officer, emphasized, “With our clear strategic roadmap, a robust pipeline of potential innovative medicines and vaccines, and a talented team laser-focused on execution, we believe we are on course to deliver significant shareholder value.” This quote highlights Pfizer’s strategic direction amidst regulatory changes.
Additionally, Pfizer is poised to provide further insights during an analyst webcast scheduled for December 17, 2024, which will explore its market access strategies and regulatory adaptations.
In summary, Pfizer’s strategic navigation of regulatory changes, supported by comprehensive Medicare data insights from CareSet, is crucial for maintaining market access and ensuring that patients can access innovative therapies, ultimately contributing to improved health outcomes.
Trump’s Tariff Policies: Implications for Pharma Profitability
According to pharma industry news, the drug industry is acutely aware of the ramifications of Trump’s tariff policies, which pose a significant threat to cost structures and supply chain stability. While the precise percentage of suggested tariffs on medicine imports may differ, the possibility of significant increases in drug prices is evident, with projections indicating that expenses could climb by as much as 10% for specific treatments. This situation is particularly concerning for companies that depend heavily on imported materials, as it may result in decreased profitability and hinder their ability to invest in research and development.
In response to these challenges, stakeholders are reassessing their supply chain strategies based on the latest pharma industry news. Many are exploring domestic manufacturing options, as evidenced by Johnson & Johnson’s recent commitment to invest $55 billion in U.S. production capabilities. This action not only reflects a broader trend among major drug companies to enhance their manufacturing presence in the U.S. but also serves as a strategic response to the pressures imposed by tariffs.
The ripple effect of these tariffs is anticipated to trickle down to patients, with medical expenses likely to rise as producers of generic oral medications can more easily transfer increased costs compared to those creating injectable drugs. As companies navigate this complex landscape, strategies for mitigating tariff impacts are becoming essential, as highlighted in pharma industry news. These may include:
- Diversifying supply sources
- Investing in local production
- Leveraging technology to optimize operations
For instance, some companies are actively seeking alternative suppliers to reduce dependency on imports, while others are investing in automation to enhance efficiency and reduce costs.
In this context, CareSet’s comprehensive healthcare information insights can empower pharmaceutical companies to make informed decisions. By utilizing data analytics, companies can identify cost-effective suppliers, optimize their supply chains, and better understand market dynamics. Expert opinions highlighted in pharma industry news underscore the urgency of addressing these tariff-related challenges. Economists warn that the financial implications of proposed tariffs could lead to a significant shift in pricing strategies, potentially reviving discussions around tying U.S. drug prices to those in other countries. As Umer Raffat, an analyst at Evercore ISI, notes, “It could backfire in a big way, and could revive a plan from Trump’s first term that ties U.S. prices to those paid in other similar countries.” As the industry adapts to these evolving conditions, the focus remains on maintaining profitability while ensuring patient access to essential medications.

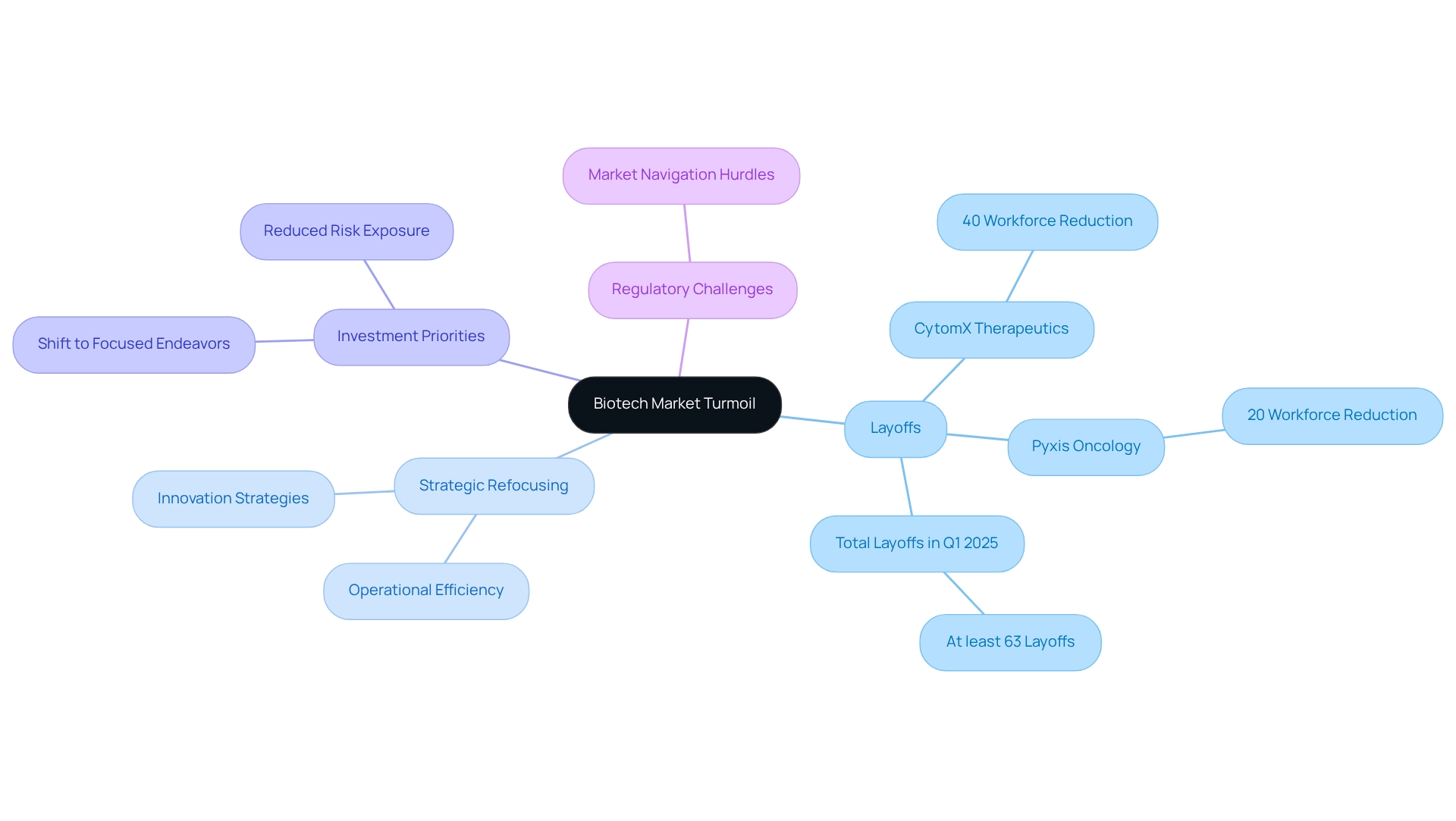
Biotech Market Turmoil: Layoffs and Uncertainty Ahead
The biotech sector is currently navigating a turbulent landscape, characterized by significant layoffs and restructuring efforts as companies respond to mounting market pressures. In the first quarter of 2025 alone, biopharmaceutical firms executed layoffs at least 63 times—an alarming statistic that underscores the urgency of the situation. Notably, CytomX Therapeutics has announced a 40% reduction in its workforce to concentrate on its lead asset. Similarly, Pyxis Oncology is streamlining operations with a 20% workforce cut, following a prior 40% reduction after acquiring Apexigen. This wave of layoffs illustrates a broader trend of capital discipline and strategic restraint within the industry.
As Ankit Kankar, an opinion writer, observes, this strategic refocusing reflects the current climate, where companies must prioritize innovation and operational efficiency to remain competitive and attract investment. The shift in investment priorities is moving from expansive research and development portfolios to more focused endeavors that promise quicker returns and reduced risk exposure.
Moreover, regulatory challenges continue to pose significant hurdles for biotech firms, complicating their ability to navigate the market effectively. The combination of these factors creates a cautious outlook for many companies, compelling them to adapt swiftly to survive in this evolving environment. For instance, firms that have successfully implemented lean operational models and agile project management strategies are better positioned to thrive amidst uncertainty. The emphasis on innovation strategies is crucial, as these approaches not only streamline operations but also foster new developments that can lead to sustainable growth.
AI Innovations: Transforming Drug Discovery and Development
In the latest pharma industry news, artificial intelligence is revolutionizing drug discovery and development, empowering drug companies to optimize processes and significantly reduce costs. By leveraging advanced machine learning algorithms and robust analytics, organizations can more effectively identify promising drug candidates and forecast their success rates in clinical trials. This shift toward AI-driven methodologies is anticipated not only to accelerate innovation but also to enhance the overall effectiveness of drug development.
Recent statistics highlight this trend: the ten-year rolling average of FDA drug approvals has soared from 25 per year in 2010 to 46 currently, illustrating how AI is facilitating this growth by streamlining the drug development process. Furthermore, since the onset of the COVID-19 pandemic, the volume of electronic communications to healthcare providers has surged by 57%, reflecting an increasing reliance on digital solutions that AI technologies can enhance within the healthcare sector.
In this context, on October 19, 2016, CareSet Systems introduced groundbreaking data science products designed to support drug introductions, incorporating features such as advanced predictive analytics and real-time data integration. This further exemplifies the diverse applications of AI within the healthcare industry. These advancements are emerging as pivotal drivers of growth and innovation. For instance, machine learning is increasingly employed to streamline clinical trial processes, resulting in improved success rates. Experts assert that AI technologies are not merely enhancing operational efficiency; they are also reshaping the strategic landscape of drug development, as reported in the latest pharma industry news.
Pharmaceutical leaders acknowledge the influence of machine learning, with many asserting that these technologies will confer a competitive advantage in the market. According to a survey by MIT Sloan Management, 87% of global organizations believe that AI technologies will provide them this edge, underscoring the critical role of AI in the healthcare industry. As AI continues to evolve, its contribution to transforming pharmaceutical processes is poised to expand, paving the way for more effective and efficient drug discovery and development.
Patient Engagement: Strategies for Improved Treatment Outcomes
Pharmaceutical firms are increasingly recognizing the essential role of consumer engagement in enhancing treatment outcomes. Strategies such as personalized communication, the integration of digital wellness tools, and comprehensive patient education initiatives are being adopted to foster stronger relationships between patients and providers. CareSet’s extensive Medicare information solutions, encompassing insights from over 62 million beneficiaries and 6 million providers, empower healthcare stakeholders with actionable information that can inform these engagement strategies. Notably, CareSet’s data reflects more than $900 billion in annual claims, underscoring the financial implications of these insights. For instance, recent statistics reveal that:
- 37% of users monitor their heart health with smartwatches
- 35% track their sleep quality and duration
This growing reliance on technology not only mirrors individuals’ proactive health management but also highlights the importance of integrating such tools into engagement strategies to promote better adherence to treatment plans.
By prioritizing client engagement, companies can significantly enhance adherence to treatment plans, which is crucial for achieving optimal health outcomes. Engaging individuals through tailored communication fosters trust and empowers them to take an active role in their healthcare journey. Furthermore, the use of digital health tools has been shown to improve treatment adherence, with numerous individuals expressing increased motivation to comply with their prescribed regimens. CareSet’s monthly Medicare updates provide insights into drug usage and treatment pathways, further assisting companies in their efforts to engage individuals effectively.
Expert insights underscore that effective client engagement strategies are vital for driving better business results, as highlighted in pharma industry news. For instance, companies that invest in consumer engagement initiatives frequently observe significant enhancements in treatment analytics and market reach. As Beth Brooks from Sanofi articulates, “If we lower the barriers for the people who have the most difficulty participating, we are lowering the barriers for everybody.” This perspective emphasizes the importance of making involvement accessible to all.
As the landscape evolves, the importance of consumer engagement in driving treatment outcomes cannot be overstated, which is a key focus for pharmaceutical market access managers in 2025, as reported in pharma industry news. To enhance your patient engagement strategies, consider leveraging CareSet’s insights to implement regular feedback mechanisms that better understand patient needs and adjust your approaches accordingly.

Conclusion
The pharmaceutical industry is experiencing a profound transformation, propelled by an increasing reliance on data-driven insights and innovative technologies. Companies such as CareSet, Roche, Johnson & Johnson, Regeneron, and Pfizer are harnessing advanced analytics and real-world evidence to navigate complex challenges, ranging from regulatory changes to market access strategies. By integrating comprehensive Medicare data and prioritizing patient engagement, these organizations are not only improving treatment outcomes but also enhancing their operational efficiency.
As healthcare spending continues to escalate, the imperative for pharmaceutical companies to leverage data insights has never been more critical. CareSet’s focus on analyzing Medicare claims data exemplifies how such information can yield a deeper understanding of treatment patterns, patient demographics, and provider networks. This strategic approach empowers companies to identify new market opportunities and optimize their strategies within an ever-evolving landscape.
Furthermore, initiatives promoting diversity in clinical trials and the integration of artificial intelligence into drug development processes are establishing new benchmarks for the industry. By prioritizing inclusivity and employing cutting-edge technologies, companies can ensure that their products adequately meet the needs of diverse patient populations while streamlining development timelines.
In conclusion, the future of the pharmaceutical industry hinges on the capacity to adapt to market dynamics through data-driven strategies. By embracing insights from organizations like CareSet and emphasizing patient engagement, pharmaceutical companies can significantly enhance their growth prospects while contributing to improved health outcomes for patients. As the industry continues to evolve, the integration of innovative data solutions will be essential for sustaining competitive advantage and fostering positive change in healthcare delivery.


